Aminal
An aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: -C(NR2)(NR2)-. (As is customary in organic chemistry, R can represent hydrogen or an alkyl group).[1]

The aminal and the hemiaminal groups are analogous to hemiacetals and acetals with nitrogen replaced by oxygen. Aminals are encountered in, for instance, the Fischer indole synthesis. Cyclic aminals are well known, being typically derived by the condensation of a diamine and an aldehyde.[2] An example (although not derived from a diamine) is hexamethylenetetramine derived from ammonia and formaldehyde.
Hemiaminal ethers
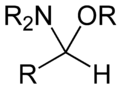

Hemiaminal ethers are also sometimes called aminals although it is discouraged by the IUPAC. The ethers have the following structure: R‴-C(NR'2)(OR")-R⁗. The glycosylamines are examples of cyclic hemiaminal ethers.
See also
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "aminals". doi:10.1351/goldbook.A00270
- Hiersemann, M. "Functions bearing two nitrogens" in Comprehensive Organic Functional Group Transformations II 2005, volume 4, 411-441. Edited by Katritzky, Alan R.; Taylor, Richard J. K. doi:10.1016/B0-08-044655-8/00075-1