Aluminized steel
Aluminized steel is steel that has been hot-dip coated on both sides with aluminium-silicon alloy. This process assures a tight metallurgical bond between the steel sheet and its aluminium coating, producing a material with a unique combination of properties possessed neither by steel nor by aluminium alone. Aluminized steel shows a better behavior against corrosion[1] and keeps the properties of the base material steel for temperature lower than 800 °C (1,470 °F). For example, it is commonly used for heat exchangers in residential furnaces, commercial rooftop HVAC units, automotive mufflers, ovens, kitchen ranges, water heaters, fireplaces, barbecue burners, and baking pans. This steel is very useful for heating things up because it transfers heat faster than most other steels.

Characteristics are defined by the exact metals and processes used.
Types
- Type 1
- Hot-dip coated with a thin layer of aluminium / silicon alloy containing 5% to 11% silicon to promote better adherence. It is intended principally for heat resisting applications and also for uses where corrosion resistance and heat are involved. Possible end uses are mufflers, furnaces, ovens, ranges, heaters, water heaters, fireplaces, and baking pans. Aluminized steel can withstand 550 °C (1,022 °F) with almost no change in the base material. But due to silicon content it develops black spot. Aluminized steel has slowly started to convert bakery trays which were previously made by galvanized or galvalume steel as it does not contain lead which is poisonous. Type 1 is also commonly found in industrial products.
- Type 2
- Hot-dip coated with commercially pure aluminum. It is intended principally for applications requiring atmospheric corrosion resistance. Type 2 may ultimately be manufactured into corrugated roofing and siding, grain bins, drying ovens, and air-conditioner condenser housings.
Properties
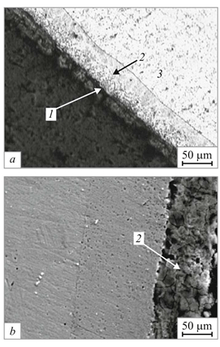
The basic structure of aluminized steel is a thin aluminium oxide layer outside, then an intermetallic layer that is a mix of aluminium, silicon, and steel, and finally a steel core.[2]
Both Type 1 and Type 2 show excellent high reflectivity characteristics. At temperatures up to 842 °C (1,548 °F), aluminized steel reflects up to 80% of heat projected onto it.[3] Aluminized steel has the ability to maintain its strength at temperatures up to 677 °C (1,251 °F). Although stainless steel is the stronger of the two, aluminized steel has a greater electrostatic surface, and can therefore reflect heat better.
Aluminized steel is highly resistant to corrosion because of the thin layers of aluminium and silicon, which keep the underlying steel from oxidizing. These thin layers also keep pit corrosion from occurring, especially during exposure to salts that affect most other metals. However, despite the good corrosion resistance of aluminized steel, if the aluminium layer is disrupted and the steel is exposed, then the steel may oxidize and corrosion may occur.
Consumption
In North America nearly 700,000 tons of aluminized steel are consumed annually.[4] Some of the common products made from aluminized steel include water heaters, ranges, furnaces, space heaters and grills.
Processing
Aluminized steel can be made using a variety of processes, cladding, hot dipping, galvanic coating, metallizing, and calorizing, but the most effective process is hot dipping. The process of hot dipping starts by cleaning the steel, then placing the steel in a bath of Al-11%Si at a temperature of 988K and shaken, then pulled out and air dried.[5] The aluminium diffuses into the steel, creating an intermetallic layer above the steel base layer, but below the outside aluminum coating. The aluminium coating is oxidized to help protect the inner steel from corrosion and further aluminium diffusion.[6] The silicon is added to the aluminium bath to create a thinner layer of aluminium on the steel. The hot dipping process is cheaper and more effective to produce aluminized steel than any other process.[7]
Uses

Aluminized steel was developed for providing more structural durability and a high yield strength in highly corrosive environments. It maintains the strength of high-alloy steel, but is cheaper to produce than high-alloy steels and thus is a preferred material for manufacturing automobile and motorcycle exhaust gas systems.[7]
Al-Si coatings are used to protect boron steel when hot pressing.
References
- ""Aluminized steel Offers Attractive Physical Characteristics For Use In Industrial Duct Construction". Sheet Metal and Air Conditioning Contractors' National Association. Retrieved 26 Feb 2011". Archived from the original on 2011-02-20. Retrieved 2011-02-26.
- Kee-Hyun, Kim. Van-Daele, Benny. Van-Tendeloo, Gusfaaf. and Jong-Kyu, Yoon. (2006). "Observations of Intermetallic Compound Formation of Hot Dip Aluminized steel". Materials Science Forum, 519-21(2), 1871-75.
- Atlas Steel - Aluminized steel
- "Archived copy". Archived from the original on 2010-09-30. Retrieved 2011-11-29.CS1 maint: archived copy as title (link)
- Rajendran, R. Venkataswamy, S. Jaikrishna, U. Gowrishankar, N. and Rajadurai, A. (2006). "Effect of Process Parameters in Hot Dip Aluminizing of Medium Carbon Steel".
- Deqing, Wang. and Ziyuan, Shi. (2003) "Formation of Al2O3 Layer on Steel". Journal of Materials Science Letters, 22(14), 1003-1006.
- Wang, Chaur. Jeng. Badaruddin, Mohd.. (2010) "The dependence of high temperature resistance of aluminized steel exposed to water-vapour oxidation". Surface and Coatings Technology, 205(5), 1200-1205.