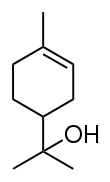
Terpineol
Terpineol is a monoterpene alcohol that has been isolated from a variety of sources such as cajuput oil, pine oil, and petitgrain oil.[2] There are four isomers: alpha-, beta-, gamma-terpineol, and terpinen-4-ol. Beta- and gamma-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with alpha-terpineol as the major constituent.
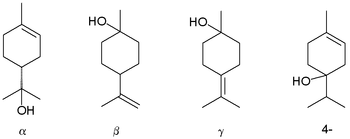 Terpineols: alpha-, beta-, gamma-, and the 4-terpineol isomer
Terpineols: alpha-, beta-, gamma-, and the 4-terpineol isomer
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-(4-Methylcyclohex-3-en-1-yl)propan-2-ol | |||
| Other names
2-(4-Methyl-1-cyclohex-3-enyl)propan-2-ol alpha-terpineol α-terpineol p-menth-1-en-8-ol α,α,4-Trimethylcyclohex-3-ene-1-methanol Terpene alcohol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| UNII | |||
| |||
| |||
| Properties | |||
| C10H18O | |||
| Molar mass | 154.253 g·mol−1 | ||
| Appearance | Colorless liquid[1] | ||
| Density | 0.93 g/cm3[1] | ||
| Melting point | −35.9 to −28.2 °C (−32.6 to −18.8 °F; 237.2 to 245.0 K)[1] (mixture of isomers) | ||
| Boiling point | 214–217 °C (417–423 °F; 487–490 K)[1] (mixture of isomers) | ||
| 2.42 g/L[1] | |||
| −111.9·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 88 °C (190 °F; 361 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Terpineol has a pleasant odor similar to lilac and is a common ingredient in perfumes, cosmetics, and flavors. α-terpineol is one of the two most abundant aroma constituents of lapsang souchong tea; the α-terpineol originates in the pine smoke used to dry the tea.[3] (+)-α-terpineol is a chemical constituent of skullcap.
Synthesis
Although it is naturally occurring, terpineol is commonly manufactured from the more readily available alpha-pinene.
In one study, an alternative route starting from limonene was demonstrated:[4]
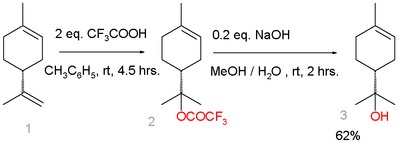 Terpineol synthesis from limonene
Terpineol synthesis from limonene
Limonene is reacted with trifluoroacetic acid in a Markovnikov addition to a trifluoroacetate intermediate, which is easily hydrolyzed with sodium hydroxide to α-terpineol with 7% selectivity. Side-products are β-terpineol in a mixture of the cis-isomer, the trans-isomer, and 4-terpineol.
References
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Merck Index, 11th Edition, 9103
- Shan-Shan Yao; Wen-Fei Guo; Yi Lu; Yuan-Xun Jiang (2005). "Flavor Characteristics of Lapsang Souchong and Smoked Lapsang Souchong, a Special Chinese Black Tea with Pine Smoking Process". Journal of Agricultural and Food Chemistry. 53 (22): 8688–93. doi:10.1021/jf058059i. PMID 16248572.
- Yuasa, Yoshifumi; Yuasa, Yoko (2006). "A Practical Synthesis of d-α-Terpineol via Markovnikov Addition of d-limonene Using Trifluoroacetic Acid". Organic Process Research & Development. 10 (6): 1231–1232. doi:10.1021/op068012d.
External links
- MSDS for alpha-terpineol