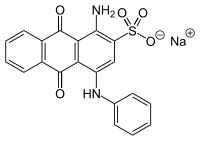
Acid Blue 25
Acid Blue 25 (C20H13N2NaO5S) is an acid dye that is water-soluble and anionic and used for adsorption researches.[1] The structure is an anthraquinone.[2]
 | |
| Names | |
|---|---|
| IUPAC name
Sodium 1-amino-4-anilino-9,10-dioxoanthracene-2-sulfonate | |
| Other names
2-Anthraquinonesulfonic acid, Acid Blue 25 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.026.426 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H13N2NaO5S | |
| Molar mass | 416.38 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties and applications
Acid Blue 25 is powder-like and poorly soluble in water. The dye is soluble in solvents such as acetone and ethanol.[2]
Use
Acid Blue 25 is used for dyeing wool, silk and mixed fabric and printing them in a direct method.[2]
The dye is extensively utilized to color leather, paper, cellulose, and PC blends during the manufacturing process.

The hue of acid Blue 25 is close to dark blue.[3]
gollark: No, the chaos communication congress™.
gollark: It is a thing, I've heard of it before.
gollark: 1. this is not really "spying stuff"2. where *should* I be looking, exactly?3. ...
gollark: ...
gollark: There were, according to the Wikipedia page, actually some security flaws found.
References
- "Acid Blue 25 (CAS 6408-78-2)". Archived from the original on 2014-03-11. Retrieved 2014-03-11.
- "World dye variety".
- "PHYSICAL AND CHEMICAL PROPERTIES OF C.I. ACID BLUE 25".
External links
- ACID BLUE 25 Basic information chemicalbook.com
- Acid Blue 25 sigmaaldrich.com
- Ghodbane, Houria; Hamdaoui, Oualid (2010). "Decolorization of antraquinonic dye, C.I. Acid Blue 25, in aqueous solution by direct UV irradiation, UV/H2O2 and UV/Fe(II) processes". Chemical Engineering Journal. 160: 226–231. doi:10.1016/j.cej.2010.03.049.
- TREATMENT OF ACID BLUE 25 CONTAINING WASTEWATERS BY ELECTROCOAGULATION scientificbulletin.upb
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
