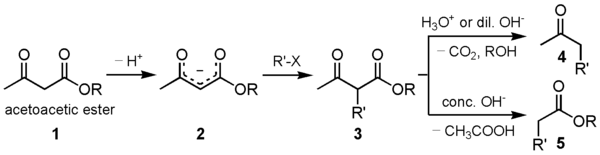
Acetoacetic ester synthesis
Acetoacetic ester synthesis is a chemical reaction where ethyl acetoacetate is alkylated at the α-carbon to both carbonyl groups and then converted into a ketone, or more specifically an α-substituted acetone. This is very similar to malonic ester synthesis.
Mechanism
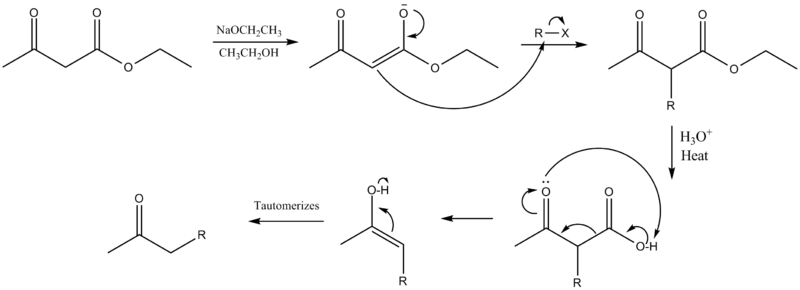
A strong base deprotonates the dicarbonyl α-carbon. This carbon is preferred over the methyl carbon because the formed enolate is conjugated and thus resonance stabilized. The carbon then undergoes nucleophilic substitution. When heated with aqueous acid, the newly alkylated ester is hydrolyzed to a β-keto acid, which is decarboxylated to form a methyl ketone.[1][2]
gollark: No, that was a while ago.
gollark: The annoying people who write curricula here moved matrices off into further maths, where most people don't do them, for some reason.
gollark: I found this very "fun" exchange thanks to occasionally-usable Discord search. https://discord.com/channels/424394851170385921/453931191658348545/856653348232757279
gollark: The sky blueness thing is basically just because the atmosphere scatters blue a lot more than other frequencies, right?
gollark: I see. I'll consult the records.
See also
References
- Smith, Janice Gorzynski. Organic Chemistry: Second Ed. 2008. pp 905–906
- Acetoacetic Ester Synthesis – Alkylation of Enolates | PharmaXChange.info
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.