Acetic formic anhydride
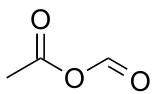
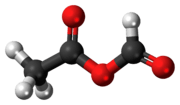
Acetic formic anhydride is an organic compound with the chemical formula C
3H
4O
3 and a structural formula of H
3C-(C=O)-O-(C=O)H. It can be viewed as the mixed anhydride of acetic acid and formic acid.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acetic formic anhydride | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4O3 | |
| Molar mass | 88.062 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Acetic formic anhydride can be produced by reacting sodium formate with acetyl chloride in anhydrous diethyl ether between 23–27 °C.[2] It can also be prepared by the reaction of acetic anhydride and formic acid at 0 °C.
Applications
Acetic formic anhydride is a formylation agent for amines, amino acids, and alcohols. It is also a starting material for other compounds such as formyl fluoride.[2]
Acetic formic anhydride is used in the synthesis of quazodine.[3]
gollark: ... why is there no built-in way to truncate strings in-place...
gollark: Exciting! Every change I make causes an exciting new compile error.
gollark: Oh no. My code CANNOT satisfy Ferris.
gollark: ÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆ ÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆ ÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆÆ
gollark: Countermeasures may be required.
References
- "Formyl acetate - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Krimen, Lewis I. (1970). "Acetic Formic Anhydride". Organic Syntheses. 50: 1. doi:10.15227/orgsyn.050.0001.
- U.S. Patent 3,248,292
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.