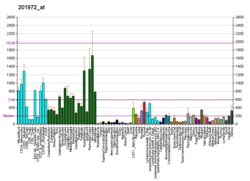
ATP6V1A
V-type proton ATPase catalytic subunit A is an enzyme that in humans is encoded by the ATP6V1A gene.[5][6]
This gene encodes a component of vacuolar ATPase (V-ATPase), a multisubunit enzyme that mediates acidification of eukaryotic intracellular organelles. V-ATPase dependent organelle acidification is necessary for such intracellular processes as protein sorting, zymogen activation, receptor-mediated endocytosis, and synaptic vesicle proton gradient generation. V-ATPase is composed of a cytosolic V1 domain and a transmembrane V0 domain. The V1 domain consists of three A and three B subunits, two G subunits plus the C, D, E, F, and H subunits. The V1 domain contains the ATP catalytic site. The V0 domain consists of five different subunits: a, c, c', c", and d. Additional isoforms of many of the V1 and V0 subunit proteins are encoded by multiple genes or alternatively spliced transcript variants. This encoded protein is one of two V1 domain A subunit isoforms and is found in all tissues. Transcript variants derived from alternative polyadenylation exist.[6]
References
- GRCh38: Ensembl release 89: ENSG00000114573 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000052459 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- van Hille B, Richener H, Evans DB, Green JR, Bilbe G (May 1993). "Identification of two subunit A isoforms of the vacuolar H(+)-ATPase in human osteoclastoma". J Biol Chem. 268 (10): 7075–80. PMID 8463241.
- "Entrez Gene: ATP6V1A ATPase, H+ transporting, lysosomal 70kDa, V1 subunit A".
External links
- Human ATP6V1A genome location and ATP6V1A gene details page in the UCSC Genome Browser.
Further reading
- Finbow ME, Harrison MA (1997). "The vacuolar H+-ATPase: a universal proton pump of eukaryotes". Biochem. J. 324 (Pt 3): 697–712. doi:10.1042/bj3240697. PMC 1218484. PMID 9210392.
- Stevens TH, Forgac M (1998). "Structure, function and regulation of the vacuolar (H+)-ATPase". Annu. Rev. Cell Dev. Biol. 13: 779–808. doi:10.1146/annurev.cellbio.13.1.779. PMID 9442887.
- Nelson N, Harvey WR (1999). "Vacuolar and plasma membrane proton-adenosinetriphosphatases". Physiol. Rev. 79 (2): 361–85. doi:10.1152/physrev.1999.79.2.361. PMID 10221984.
- Forgac M (1999). "Structure and properties of the vacuolar (H+)-ATPases". J. Biol. Chem. 274 (19): 12951–4. doi:10.1074/jbc.274.19.12951. PMID 10224039.
- Kane PM (1999). "Introduction: V-ATPases 1992-1998". J. Bioenerg. Biomembr. 31 (1): 3–5. doi:10.1023/A:1001884227654. PMID 10340843.
- Wieczorek H, Brown D, Grinstein S, et al. (1999). "Animal plasma membrane energization by proton-motive V-ATPases". BioEssays. 21 (8): 637–48. doi:10.1002/(SICI)1521-1878(199908)21:8<637::AID-BIES3>3.0.CO;2-W. PMID 10440860.
- Nishi T, Forgac M (2002). "The vacuolar (H+)-ATPases--nature's most versatile proton pumps". Nat. Rev. Mol. Cell Biol. 3 (2): 94–103. doi:10.1038/nrm729. PMID 11836511.
- Kawasaki-Nishi S, Nishi T, Forgac M (2003). "Proton translocation driven by ATP hydrolysis in V-ATPases". FEBS Lett. 545 (1): 76–85. doi:10.1016/S0014-5793(03)00396-X. PMID 12788495.
- Morel N (2004). "Neurotransmitter release: the dark side of the vacuolar-H+ATPase". Biol. Cell. 95 (7): 453–7. doi:10.1016/S0248-4900(03)00075-3. PMID 14597263.
- van Hille B, Richener H, Green JR, Bilbe G (1995). "The ubiquitous VA68 isoform of subunit A of the vacuolar H(+)-ATPase is highly expressed in human osteoclasts". Biochem. Biophys. Res. Commun. 214 (3): 1108–13. doi:10.1006/bbrc.1995.2400. PMID 7575517.
- Hu RM, Han ZG, Song HD, et al. (2000). "Gene expression profiling in the human hypothalamus-pituitary-adrenal axis and full-length cDNA cloning". Proc. Natl. Acad. Sci. U.S.A. 97 (17): 9543–8. doi:10.1073/pnas.160270997. PMC 16901. PMID 10931946.
- Chang SY, Park SG, Kim S, Kang CY (2002). "Interaction of the C-terminal domain of p43 and the alpha subunit of ATP synthase. Its functional implication in endothelial cell proliferation". J. Biol. Chem. 277 (10): 8388–94. doi:10.1074/jbc.M108792200. PMID 11741979.