8-Azaguanine
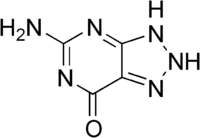
8-Azaguanine is a purine analog with the chemical formula C4H4N6O. It has been widely studied for its biological activity.[5] It shows antineoplastic activity and has been used in the treatment of acute leukemia.[2]
 | |
| Names | |
|---|---|
| IUPAC names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.681 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4N6O | |
| Molar mass | 152.117 g·mol−1 |
| Appearance | white to off-white crystalline powder[4] |
| Density | 2.64 g/cm³ |
| Melting point | > 300 °C (decomp.) |
| Insoluble | |
| Hazards | |
| Flash point | 129.1 °C (264.4 °F; 402.2 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use in chemotherapy
The compound closely resembles guanine and appears to be competitive with it in the metabolism of living organisms.[6] It has been shown to cause retardation of some malignant neoplasms when administered to tumors in animals.[6] 8-Azaguanine was the first purine analogue discovered to inhibit experimental tumors in mice.[7]
Synonyms
|
|
gollark: Write all your code in brainf***.
gollark: Er, yes, most languages.
gollark: Rust.
gollark: The bad error handling?
gollark: <@301092081827577866> go ≈ bad
References
- "Azaguanine - Compound Summary (Descriptors)". National Center for Biotechnology Information. 27 March 2005. Retrieved 2009-03-03.
- "8-azaguanine". Mondofacto. 12 December 1998. Retrieved 2009-03-03.
- http://www.chemindustry.com/chemicals/747854.html Retrieved on 2009-03-03.
- "8-AZAGUANINE". ChemicalLAND21.com. Retrieved 2009-03-03.
- Tong, George L.; Lee, William W.; Goodman, Leon; Frederiksen, Sune (1965). "Synthesis of some 2′-deoxyribosides of 8-azaadenine". Archives of Biochemistry and Biophysics. University of California: Elsevier. 112 (1): 76. doi:10.1016/0003-9861(65)90012-3.
- Colsky, J.; Meiselas, E.L.; Rosen, J.S.; Schulman, I. (1955). "Response of patients with leukemia to 8-azaguanine" (PDF). Blood. 10 (5): 482–92. doi:10.1182/blood.V10.5.482.482. PMID 14363328.
- Timmis, G.M.; Williams, Donald Charles (1967). "Chemotherapy of Cancer: the Antimetabolite Approach: The Antimetabolite Approach". University of Michigan: Butterworths: 36. Cite journal requires
|journal=(help) - http://www.chemcas.com/msds/cas/msds137/134-58-7.asp Retrieved on 2009-03-03.
- "Azaguanine - Compound Summary (Synonyms)". National Center for Biotechnology Information. 27 March 2005. Retrieved 2009-03-03.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.