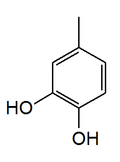
4-Methylcatechol
4-Methylcatechol is a chemical compound. It is a component of castoreum, the exudate from the castor sacs of the mature beaver.[1]
 | |
| Names | |
|---|---|
| IUPAC name
4-Methylbenzene-1,2-diol | |
| Other names
4-Methyl-1,2-dihydroxybenzene 3,4-Dihydroxytoluene Homocatechol 4-Methyl-1,2-benzenediol Homopyrocatechol p-Methylcatechol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.559 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C7H8O2 | |
| Molar mass | 124.13 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metabolism
The enzyme cis-1,2-dihydroxy-4-methylcyclohexa-3,5-diene-1-carboxylate dehydrogenase uses cis-1,2-dihydroxy-4-methylcyclohexa-3,5-diene-1-carboxylate and NAD(P)+ to produce 4-methylcatechol, NADH, NADPH and CO2.[2]
Related compounds
Members of the monocot subfamily Amaryllidoideae present a unique type of alkaloids, the norbelladine alkaloids, which are 4-methylcatechol derivatives combined with tyrosine. They are responsible for the poisonous properties of a number of the species. Over 200 different chemical structures of these compounds are known, of which 79 or more are known from Narcissus alone.[3]
Production
The brand of low-temperature coke used as a smokeless fuel Coalite obtains homocatechol from ammoniacal liquor by solvent extraction, distillation and crystallisation.
See also
References
- Pheromonal activity of single castoreum constituents in beaver,Castor canadensis., Müller-Schwarze, D and Houlihan, P.W., Journal of Chemical Ecology, April 1991, Volume 17, Number 4, Springer Netherlands, doi:10.1007/BF00994195
- Whited GM, McCombie WR, Kwart LD, Gibson DT (1986). "Identification of cis-diols as intermediates in the oxidation of aromatic acids by a strain of Pseudomonas putida that contains a TOL plasmid". J. Bacteriol. 166 (3): 1028–39. PMC 215228. PMID 3711022.
- Martin, S.F. 1987. The Amaryllidaceae Alkaloids. In.: Arnold Brossi (ed.) The Alkaloids, Chapter 3. Academic Press.