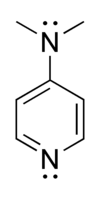
4-Dimethylaminopyridine
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH3)2NC5H4N. This colourless solid is of interest because it is more basic than pyridine, owing to the resonance stabilisation from the NMe2 substituent.
 | |
| Names | |
|---|---|
| IUPAC names
N,N-Dimethylpyridin-4-amine Dimethyl(pyridin-4-yl)azane Dimethyl(pyridin-4-yl)amine | |
| Preferred IUPAC name
N,N-Dimethylpyridin-4-amine | |
| Other names
4-(Dimethylamino)pyridine N,N-Dimethyl-4-aminopyridine DMAP 4-Dimethylaminopyridine 4-(Dimethylamino)azine N,N-dimethyl-4-aminoazine 4-(Dimethylamino)azabenzene N,N-Dimethyl-4-aminoazabenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.049 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H10N2 | |
| Molar mass | 122.17 g/mol |
| Appearance | white solid |
| Melting point | 110 to 113 °C (230 to 235 °F; 383 to 386 K) |
| Boiling point | 162 °C (324 °F; 435 K) at 50 mmHg |
| Acidity (pKa) | 9.6 in water, 17.95 (pKa of conjugate acid in acetonitrile)[1] |
| Hazards | |
| Safety data sheet | [2] |
| GHS pictograms |  |
| GHS Signal word | Danger |
GHS hazard statements |
H301, H310, H315, H319, H335[2] |
| P280, P305+351+338, P337+313[2] | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
deer mice: oral, 450 mg/kg[3] mice: oral, 350 mg/kg/day[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because of its basicity, DMAP is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich rearrangement, Staudinger synthesis of β-lactams and many more. Chiral DMAP analogues are used in kinetic resolution experiments of mainly secondary alcohols and Evans auxiliary type amides.[4][5][6]
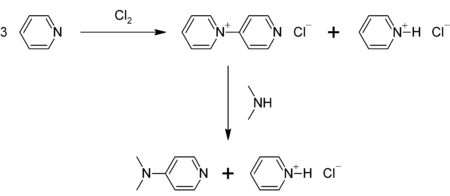
Preparation
DMAP can be prepared in a two-step procedure from pyridine, which is first oxidized to 4-pyridylpyridinium cation. This cation then reacts with dimethylamine:[7]
Esterification catalyst
In the case of esterification with acetic anhydrides the currently accepted mechanism involves three steps. First, DMAP and acetic anhydride react in a pre-equilibrium reaction to form an ion pair of acetate and the acetylpyridinium ion. In the second step the alcohol adds to the acetylpyridinium, and elimination of pyridine forms an ester. Here the acetate acts as a base to remove the proton from the alcohol as it nucleophilically adds to the activated acylpyridinium. The bond from the acetyl group to the catalyst gets cleaved to generate the catalyst and the ester. The described bond formation and breaking process runs synchronous concerted without the appearance of a tetrahedral intermediate. The acetic acid formed will then protonate the DMAP. In the last step of the catalytic cycle the auxiliary base (usually triethylamine or pyridine) deprotonates the protonated DMAP, reforming the catalyst. The reaction runs through the described nucleophilic reaction pathway irrespective of the anhydride used, but the mechanism changes with the pKa value of the alcohol used. For example, the reaction runs through a base-catalyzed reaction pathway in the case of a phenol. In this case, DMAP acts as a base and deprotonates the phenol, and the resulting phenolate ion adds to the anhydride.[8]
Safety
DMAP has a relatively high toxicity and is particularly dangerous because of its ability to be absorbed through the skin. It is also corrosive.[9]
References
- Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- Sigma-Aldrich Co., 4-(Dimethylamino)pyridine. Retrieved on 2015-09-03.
- Nachtergael, Amandine; Coulembier, Olivier; Dubois, Philippe; Helvenstein, Maxime; Duez, Pierre; Blankert, Bertrand; Mespouille, Laetitia (9 February 2015). "Organocatalysis Paradigm Revisited: Are Metal-Free Catalysts Really Harmless?". Biomacromolecules. 16 (2): 507–514. doi:10.1021/bm5015443. PMID 25490408.
- Donald J Berry; Charles V Digiovanna; Stephanie S Metrick; Ramiah Murugan (2001). "Catalysis by 4-dialkylaminopyridines". Arkivoc: 201–226. Archived from the original on 2007-09-27. Retrieved 2006-11-27.
- Höfle, G.; Steglich, W.; Vorbrüggen, H. (1978). "4-Dialkylaminopyridines as Highly Active Acylation Catalysts". Angew. Chem. Int. Ed. Engl. 17 (8): 569–583. doi:10.1002/anie.197805691.
- Ryan P. Wurz (2007). "Chiral Dialkylamine Catalysts in Asymmetric Synthesis". Chem. Rev. 107 (12): 5570–5595. doi:10.1021/cr068370e. PMID 18072804.
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- S. Xu; I. Held; B. Kempf; H. Mayr; Wolfgang Steglich; H. Zipse (2005). "The DMAP-Catalyzed Acetylation of Alcohols - A Mechanistic Study (DMAP = 4-(dimethylamino)-pyridine)". Chem. Eur. J. 11 (16): 4751–4757. doi:10.1002/chem.200500398. PMID 15924289.
- DMAP MSDS - Fischer Science
Further reading
- B. Neises; W. Steglich (1990). "Esterification of Carboxylic Acids with Dicyclohexylcarbodiimide/4-Dimethylaminopyridine: tert-Butyl Ethyl Fumarate". Organic Syntheses.; Collective Volume, 7, p. 93
- I. Held; P. von den Hoff; D. S. Stephenson; H. Zipse (2008). "Domino Catalysis in the Direct Conversion of Carboxylic Acids to Esters". Adv. Synth. Catal. 11/12 (11–12): 1891–1900. doi:10.1002/adsc.200800268.