4-Chlorophenol
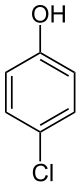
4-Chlorophenol is an organic compound with the formula ClC6H4OH. It is one of three monochlorophenol isomers. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Its pKa is 9.14.
 | |
| Names | |
|---|---|
| IUPAC name
4-Chlorophenol | |
| Other names
p-Chlorophenol | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 507004 | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.003.094 |
| EC Number |
|
| 2902 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2020 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5ClO | |
| Molar mass | 128.56 g·mol−1 |
| Appearance | White solid |
| Density | 1.31 g/cm3 |
| Melting point | 42.8 °C (109.0 °F; 315.9 K) |
| Boiling point | 217 °C (423 °F; 490 K) |
| 27.1 g/L | |
Refractive index (nD) |
1.5579 |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H290, H301, H302, H312, H314, H318, H332, H411 |
| P234, P260, P261, P264, P270, P271, P273, P280, P301+310, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P390, P391, P404 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reaction
It is prepared by chlorination of phenol, preferably in polar solvents, which tends to yield the 4-chloro derivative. Direct chlorination of molten phenol favors the formation of 2-chlorophenol.[1]
It once was produced on a large scale as a precursor to hydroquinone.[1] It is a classic precursor, upon reaction with phthalic anhydride, to quinizarin.[2] The commercial dye quinizarin is produced by the reaction of phthalic anhydride and 4-chlorophenol followed by hydrolysis of the chloride.[3]
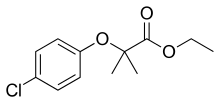
Clofibrate, a drug for controlling the high cholesterol and triacylglyceride level in the blood, is derived from 4-chlorophenol.
gollark: That would be inefficient.
gollark: Ours don't.
gollark: Advanced!
gollark: Did you know? By symmetry, bees approach me.
gollark: Not that it particularly needs one.
References
- François Muller; Liliane Caillard (2011). "Chlorophenols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_001.pub2.
- Bigelow, L. A.; Reynolds, H. H. (1926). "Quinizarin". Org. Synth. 6: 78. doi:10.15227/orgsyn.006.0078.
- Bien, H.-S.; Stawitz, J.; Wunderlich, K. "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.