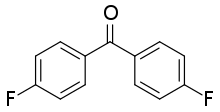
4,4'-Difluorobenzophenone
4,4’-Difluorobenzophenone is an organic compound with the formula of (FC6H4)2CO. This colorless solid is commonly used as a precursor to PEEK, or polyetherether ketone, a so-called high performance polymer. Because PEEK is resistant to attack, it is commonly used in carbon fiber coatings and cable insulation.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Bis(4-fluorophenyl)methanone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.879 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H8OF2 | |
| Molar mass | 218.20 g/mol |
| Appearance | Colorless Solid |
| Melting point | 107.5 to 108.5 °C (225.5 to 227.3 °F; 380.6 to 381.6 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H315, H319, H335, H411 |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
4,4’-Difluorobenzophenone is prepared by the acylation of fluorobenzene with p-fluorobenzoyl chloride. The conversion is typically conducted in the presence of an aluminium chloride catalyst in a petroleum ether solvent.[1]
- FC6H4C(O)Cl + C6H5F → (FC6H4)2CO + HCl
Uses
The polymer PEEK is generated from the reaction of 4,4'-difluorobenzophenone with the salts of 1,4-benzenediol.[2]
- C6H4(ONa)2 + (FC6H4)2CO → 1/n[(C6H4O2)(C13H8O)]n + 2 NaF
gollark: I've been considering bots, and they have some advantages:- they can respond faster than humans, probably- they can process vast amounts of financial databut some disadvantages:- they can't practically actually react to the content of a meme, only some metadata- I think there's comment rate limiting so they can't post that often
gollark: Hmm, yes, and it's more based on "popular meme creator who pings someone on an important server" than "good meme", I guess.
gollark: I suppose the profitable thing to do would be... to try and create interesting meme templates?
gollark: So buy shares in organic crystal meth, you're saying.
gollark: But their fortunes ultimately depend on the memes they invest in, so that doesn't work.
References
- R.D. Dunlop and John H. Gardner (1933). "Preparation of Fluorbenzophenones". J. Am. Chem. Soc. 55 (4): 1665–1666. doi:10.1021/ja01331a058.
- David Parker, Jan Bussink, Hendrik T. van de Grampe, Gary W. Wheatley, Ernst-Ulrich Dorf, Edgar Ostlinning, Klaus Reinking "Polymers, High-Temperature" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.