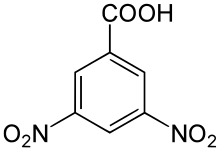
3,5-Dinitrobenzoic acid
3,5-Dinitrobenzoic acid is an organic chemical that is an important corrosion inhibitor and is also used in photography. This aromatic compound is used by chemists to identify alcohol components in esters and in the fluorometric analysis of creatinine.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,5-Dinitrobenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 1914286 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.501 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H4O6N2 | |
| Molar mass | 212.118 g/mol |
| Appearance | Yellow or colourless crystals |
| Melting point | 205 to 207 °C (401 to 405 °F; 478 to 480 K) |
| Acidity (pKa) | 2.82 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H315, H319, H335, H413 |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
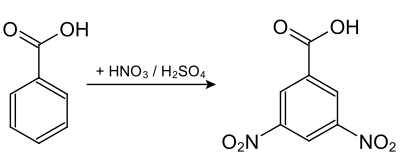
3,5-dinitrobenzoic acid is obtained from benzoic acid by the nitration reaction with nitric acid in the presence of concentrated sulfuric acid.[1][2]
The nitration can also be started with 3-nitrobenzoic acid, which leads to yields of approximately 98 %.[3]
Properties
3,5-Dinitrobenzoic acid is an odorless, yellowish solid. Due to the mesomeric effect of the two nitro groups, it is more acidic (pKa = 2.82) than benzoic acid (pKa = 4.20) and 3-nitrobenzoic acid (pKa = 3.47).
Uses
3,5-Dinitrobenzoic acid finds use in the identification of various organic substances, especially alcohols, by derivatization. For such an analysis, the substance to be analyzed is reacted with 3,5-dinitrobenzoic acid in the presence of sulfuric acid in order to form a derivate. By doing so, many substances that are liquid or have a low melting point are converted to easily crystallized derivates with sharp melting points. For instance, alcohols can be identified by the melting point of their esters with 3,5-dinitrobenzoic acid. This method is also applicable to a large number of amines.
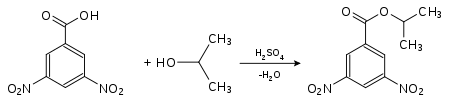 Identification of isopropanol as a derivate of 3,5-dinitrobenzoic acid:
Identification of isopropanol as a derivate of 3,5-dinitrobenzoic acid:
3,5-dinitrobenzoic acid-2-propylester (mp.: 123 °C[4]).
Compared to 4-nitrobenzoic acid, another acid that is used similarly, derivates of 3,5-dinitrobenzoic acid have higher melting points, so that it is preferred when the 4-nitrobenzoic acid derivate has a melting point too low to be accurately identified.[4]
For more sensitive compounds, the reaction is carried out using the acid chloride, 3,5-dinitrobenzoyl chloride. This allows for example the identification of amino acids.[1]
References
- Saunders, B. C.; Stacey, G. J.; Wilding, I. G. E. (1942). "The Preparation of 3:5-Dinitrobenzoic Acid and 3:5-Dinitrobenzoyl Chloride – Observations on the Acylation of Amino-acids by means of 3:5-Dinitrobenzoyl Chloride and certain other Acid Chlorides". Biochem. J. 36 (3–4): 368–375. PMC 1265703. PMID 16747534.
- R. Q. Brewster; Bill Williams; Ross Phillips (1942). "3,5-Dinitrobenzoic Acid". Organic Syntheses. 22: 48. doi:10.15227/orgsyn.022.0048.
- Lebedev, B. A.; Dolmatov, V. Yu.; Zubarev, P. S.; Latynov, N. V.; Aleksandrov, M. M.; Ponamareva, R. I (1988). "Preparation of 3,5-dinitrobenzoic acid from meta-nitrobenzoic acid". Pharmaceutical Chemistry Journal. 22 (5): 399–401. doi:10.1007/BF00769656..
- CRC Handbook of Tables for Organic Compound Identification, Third Edition, 1984, ISBN 0-8493-0303-6.
Literature
- "3,5-dinitrobenzoic acid". Combined Chemical Dictionary. Chapman and Hall/CRC Press. 2007.