Neohexene
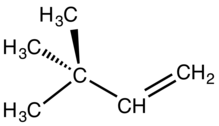
Neohexene is the organic compound with the formula (CH3)3CCH=CH2. It is a colorless liquid, with properties similar to other hexenes. It is a precursor to commercial synthetic musk perfumes.
 | |
| Names | |
|---|---|
| Other names
3,3-Dimethyl-1-butene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.361 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H12 | |
| Molar mass | 84.162 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 41 °C (106 °F; 314 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
Neohexene is prepared by ethenolysis of diisobutene, an example of a metathesis reaction:[1]
- (CH3)3C-CH=C(CH3)2 + CH2=CH2 → (CH3)3C-CH=CH2 + (CH3)2C=CH2
It is a building block to synthetic musks by its reaction with p-cymene. It is also used in the industrial preparation of terbinafine.[1]
In the study of C-H activation, neohexene is often used as a hydrogen acceptor.[2]
gollark: Yes, because it does a bit more.
gollark: Basically, in *yours*, `load` accesses the inner sandbox layer, but it didn't in mine before, so I had to bodge in a patch to load.
gollark: Line 123 and onward.
gollark: https://pastebin.com/Frv3xkB9
gollark: Yes, my sandbox also does that.
References
- Delaude, Lionel; Noels, Alfred F. (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01.
- Liu, Fuchen; Pak, Esther B.; Singh, Bharat; Jensen, Craig M.; Goldman, Alan S. (1999). "Dehydrogenation of n-Alkanes Catalyzed by Iridium "Pincer" Complexes: Regioselective Formation of α-Olefins". J. Am. Chem. Soc. 121 (16): 4086–4087. doi:10.1021/JA983460P.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.