Aminomethyl propanol
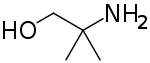
Aminomethyl propanol is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-2-methylpropan-1-ol | |
| Other names
Isobutanol-2-amine; Aminoisobutanol; 2-Amino-2-methyl-1-propanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.282 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H11NO | |
| Molar mass | 89.138 g·mol−1 |
| Density | 0.934 g/cm3 |
| Melting point | 30–31 °C (86–88 °F; 303–304 K) |
| Boiling point | 165.5 °C (329.9 °F; 438.6 K) |
| Miscible | |
| Solubility in alcohols | Soluble |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Aminomethyl propanol can be produced by the hydrogenation of 2-aminoisobutyric acid or its esters.
Uses
Aminomethyl propanol is used for the preparation of buffer solutions.[2] It is a component of the drugs ambuphylline and pamabrom. It is also used in cosmetics.[1]
It is a precursor to oxazolines via its reaction with acyl chlorides.[4] Via sulfation of the alcohol, the compound is also a precursor to 2,2-dimethylaziridine.[5]
gollark: Three headeds, anyone?
gollark: _ponders two-headed xenowyrms_
gollark: Yes, that one.
gollark: I saw a trade up *with* a 2G SAltkin asking for a ND, but I think someone else offered one before me.
gollark: Somewhere on my todo list is automating that, which ought to eventually be practical.
References
- "Aminomethyl-propanol". Cosmetics Info. Retrieved 14 August 2014.
- "2-Amino-2-methyl-1-propanol". Chemical Book. Retrieved 14 August 2014.
- Bougie, Francis; Iliuta, Maria (2012-02-14). "Sterically Hindered Amine-Based Absorbents for the Removal of CO2 from Gas Streams". J Chem Eng Data. 57: 635–669. doi:10.1021/je200731v.
- Albert I. Meyers, Mark E. Flanagan (1993). "2,2'-Dimethoxy-6-Formylbiphenyl". Org. Synth. 71: 107. doi:10.15227/orgsyn.071.0107.CS1 maint: uses authors parameter (link)
- Kenneth N. Campbell, Armiger H. Sommers, Barbara K. Campbell, Lee Irvin Smith, Oliver H. Emerson, D. E. Pearson, J. F. Baxter, K. N. Carter (1947). "Tert-butylamine". Org. Synth. 27: 12. doi:10.15227/orgsyn.027.0012.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
