2-Pyrone
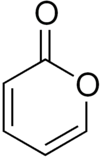
2-Pyrone (α-pyrone or pyran-2-one) is an unsaturated cyclic chemical compound with the molecular formula C5H4O2. It is isomeric with 4-pyrone.
 | |
| Names | |
|---|---|
| IUPAC name
Pyran-2-one | |
| Other names
α-Pyrone 2-Pyranone 2H-Pyran-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.264 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H4O2 | |
| Molar mass | 96.085 g·mol−1 |
| Density | 1.197 g/mL |
| Boiling point | 102 to 103 °C (216 to 217 °F; 375 to 376 K) at 20 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Pyrone is used in organic synthesis as a building block for more complex chemical structures because it may participate in a variety of cycloaddition reactions to form bicyclic lactones. For example, it readily undergoes Diels-Alder reactions with alkynes producing, upon loss of carbon dioxide, substituted benzenes.[2] The Gogte Synthesis (1938) is a method for the alkylation of certain pyrones with acid chlorides.
Derivatives
The most common natural products containing a 2-pyrone are the bufanolides and kavalactones. Oxovitisin A, a pyranoanthocyanin found in wine, also contains a 2-pyrone element.
6-Amyl-α-pyrone (6PP) is a derivative of 2-pyrone, found in animal foods and heated beef.[3] Due to its good organoleptic properties with coconut aroma, it is used as flavor enhancer in the food industry. Biologically, it is produced by Trichoderma species via solid state fermentation.[4]
Derivatives of 2-pyrone play a role as signalling molecules in bacterial communication, similar to quorum sensing. Cells with LuxR-type receptors, but lacking its homolog LuxI (and thus unable to produce N-acylhomoserine lactone QS signaling molecules) are known as LuxR "solos", to which pyrones bind as ligands facilitating cell-cell communication.[5]
See also
References
- 2H-Pyran-2-one at Sigma-Aldrich
- Woodard BT, Posner G H (1999). "Recent Advances in Diels-Alder Cycloadditions Using 2-Pyrones". Advances in Cycloaddition. 5: 47–83.
- CID 33960 from PubChem
- Ramos, Aline de Souza; Fiaux, Sorele Batista; Leite, Selma Gomes Ferreira (2008). "Production of 6-pentyl-α-pyrone by trichoderma harzianum in solid-state fermentation". Brazilian Journal of Microbiology. 39 (4): 712. doi:10.1590/S1517-83822008000400022. PMC 3768464. PMID 24031295.
- Brachmann, Alexander; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H. B.; Heermann, R. (14 July 2013). "Pyrones as bacterial signaling molecules". Nature Chemical Biology. Nature Publishing Group. 9 (9): 573–578. doi:10.1038/nchembio.1295. PMID 23851573.