2-Pyridone (data page)
This page provides supplementary chemical data on 2-pyridone.
Analytical data
NMR spectroscopy
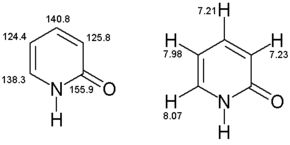
1H-NMR
1H-NMR (400 MHz, CD3OD): /ρ = 8.07 (dd,3J = 2.5 Hz,4J = 1.1 Hz, 1H, C-6), 7.98 (dd,3J = 4.0 Hz,3J = 2.0 Hz, 1H, C-3), 7.23 (dd,3J = 2.5 Hz,3J = 2.0 Hz, 1H, C-5), 7.21 (dd,3J = 4.0 Hz,4J = 1.0 Hz, 1H, C-4).
13C-NMR
(100.57 MHz, CD3OD): ρ = 155.9 (C-2), 140.8 (C-4), 138.3 (C-6), 125.8 (C-3), 124.4 (C-5)
UV/Vis spectroscopy
(MeOH):νmax (lg ε) = 226.2 (0.44), 297.6 (0.30).
IR spectroscopy
(KBr): ν = 3440 cm−1–1 (br, m), 3119 (m), 3072 (m), 2986 (m), 1682 (s), 1649 (vs), 1609 (vs), 1578 (vs), 1540 (s), 1456 (m), 1433 (m), 1364 (w), 1243 (m), 1156 (m), 1098 (m), 983 (m), 926 (w), 781 (s), 730 (w), 612 (w), 560 (w), 554 (w), 526 (m), 476 (m), 451 (w).
Mass spectrometry
EI-MS (70 eV): m/z (%) = 95 (100) [M+], 67 (35) [M+ - CO], 51 (4)[C4H3+].
gollark: Aliens? Magic cranes?
gollark: So how *did* they build them if not huge amounts of slave labour?
gollark: I'm not sure how else they would have been built, with the technology of the time.
gollark: Well, yes, lots of slaves, sure.
gollark: A very quick internet search says there were indeed no bodies found there, but also that they could plausibly just have been stolen.
References
- Cox, R. H.; Bothner-By, A. A. (1969). "Proton magnetic resonance spectra of tautomeric substituted pyridines and their conjugate acids". The Journal of Physical Chemistry. 73 (8): 2465. doi:10.1021/j100842a001.
- DW Aksnes (1972). "Substituent and solvent effects in the proton magnetic resonance (PMR) spectra of six 2-substituted pyridines" (PDF). Acta Chemica Scandinavica. 26: 2255–2266. doi:10.3891/acta.chem.scand.26-2255.
- Brügel, W. (1962). "Die Kernresonanzspektren von Pyridin-Derivaten". Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie. 66 (2): 159–177. doi:10.1002/bbpc.19620660211.
- Roberts, J. D.; Von Ostwalden, P. W. (1971). "Nuclear magnetic resonance specroscopy. Proton spectra of 2-pyridones". The Journal of Organic Chemistry. 36 (24): 3792. doi:10.1021/jo00823a029.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.