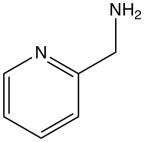
2-Picolylamine
2-Picolylamine is an organic compound with the formula H2NCH2C5H4N. A colorless liquid, it is a common bidentate ligand and a precursor to more complex multidentate ligands. It is usually prepared by hydrogenation of 2-cyanopyridine. One such complex is Baratta's catalyst RuCl2(PPh3)2(ampy) (ampy = 2-picolylamine) for transfer hydrogenation.[1] Salts of the complex [Fe(pyCH2NH2)3]2+ exhibit spin crossover behavior, whereby the complex switches from high to low spin configurations, depending on the temperature.[2]
 | |
| Names | |
|---|---|
| Other names
2-aminomethylpyridine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C6H8N2 | |
| Molar mass | 108.144 g·mol−1 |
| Density | 1.105 g/cm3 |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 203 °C (397 °F; 476 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H314, H319, H335 |
| P260, P261, P264, P271, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P337+313, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Safety
The LD50 is low, being 750 mg/kg (oral, quail).[3]
gollark: It really shouldn't be possible, but phone system bad.
gollark: BRB, calling all 10000 possible numbers.
gollark: I don't think you can strangle yourself to death.
gollark: Riiiiight.
gollark: Good job, alt!
References
- Chelucci, Giorgio; Baldino, Salvatore; Baratta, Walter (2015). "Ruthenium and Osmium Complexes Containing 2-(aminomethyl)pyridine (Ampy)-Based Ligands in Catalysis". Coordination Chemistry Reviews. 300: 29–85. doi:10.1016/j.ccr.2015.04.007.
- Gütlich, P. (2001). "Photoswitchable Coordination Compounds". Coordination Chemistry Reviews. 219-221: 839–879. doi:10.1016/S0010-8545(01)00381-2.
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.