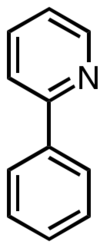
2-Phenylpyridine
2-Phenylpyridine is an organic compound with the formula C6H5C5H4N. It is a colourless viscous liquid. The compound and related derivatives have attracted interest as precursors to highly fluorescent metal complexes of possible value as organic light emitting diodes (OLEDs).[1]
 | |
| Names | |
|---|---|
| IUPAC name
2-Phenylpyridine | |
| Other names
2-Azabiphenyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.512 |
| EC Number |
|
| MeSH | C058324 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H9N | |
| Molar mass | 155.200 g·mol−1 |
| Appearance | Colorless oil |
| Density | 1.086 g/mL |
| Boiling point | 268–270 °C (514–518 °F; 541–543 K) |
| Low | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound is prepared by the reaction of phenyl lithium with pyridine:[2]
- C6H5Li + C5H5N → C6H5-C5H4N + LiH
The reaction of iridium trichloride with 2-phenylpyridine proceeds via cyclometallation to give the chloride-bridged complex:[3][4]
- 4 C6H5-C5H4N + 2 IrCl3(H2O)3 → Ir2Cl2(C6H4-C5H4N)4 + 4 HCl
This complex can be converted to the pictured tris(cyclometallated) derivative.
_Schematic.png)
Structure of Ir(C6H4-C5H4N)3
References
- Eli Zysman-Colman, ed. (2017). Iridium(III) in Optoelectronic and Photonics Applications. John Wiley & Sons. ISBN 978-1-119-00713-5.
- Evans, J. C. W.; Allen, C. F. H. (1938). "2-Phenylpyridine". Organic Syntheses. 18: 70. doi:10.15227/orgsyn.018.0070.
- Lamansky, S.; Djurovich, P.; Murphy, D.; et al. (2001). "Synthesis and Characterization of Phosphorescent Cyclometalated Iridium Complexes". Inorganic Chemistry. 40 (7): 1704–1711. doi:10.1021/ic0008969. PMID 11261983.
- Kip A. Teegardin, Jimmie D. Weaver (2018). "Preparation of Fac-Tris(2-Phenylpyridinato) Iridium(III)". Org. Synth. 95: 29. doi:10.15227/orgsyn.095.0029.
Further reading
- Zhou, Guijiang; Wong, Wai-Yeung; Yang, Xiaolong (2011). "New Design Tactics in OLEDs Using Functionalized 2-Phenylpyridine-Type Cyclometalates of Iridium(III) and Platinum(II)". Chemistry: An Asian Journal. 6 (7): 1706–1727. doi:10.1002/asia.201000928.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.