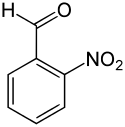
2-Nitrobenzaldehyde
2-Nitrobenzaldehyde is an organic aromatic compound containing a nitro group ortho to formyl. 2-Nitrobenzaldehyde once was produced as an intermediate in the synthesis of the popular dye Indigo.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Nitrobenzaldehyde | |||
| Other names
Nitrobenzaldehyde ortho-Nitrobenzaldehyde o-Nitrobenzaldehyde | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.206 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H5NO3 | |||
| Molar mass | 151.12 g/mol | ||
| Appearance | Pale yellow crystalline powder | ||
| Melting point | 43 °C (109 °F; 316 K) | ||
| Boiling point | 152 °C (306 °F; 425 K) | ||
| Insoluble | |||
| -68.23·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Harmful, Potentially mutagenic | ||
| R-phrases (outdated) | R36 R37 R38 R41 | ||
| S-phrases (outdated) | S26 S28 | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
The main routes to nitrobenzaldehyde begin with the nitration of styrene and cinnamic acid followed by the conversions of the resulting 2-nitrostyrene and 2-nitrocinnamic acids. Cinnamaldehyde can also be nitrated, e.g., in a solution of acetic anhydride in acetic acid, in high-yield to 2-nitrocinnamaldehyde.[3] This compound is then oxidized to 2-nitrocinnamic acid, which is decarboxylated to the 2-nitrostyrene. The vinyl group can be oxidized in a number of different ways to yield 2-nitrobenzaldehyde.[4]
In one synthetic process, toluene is mono-nitrated at cold temperatures to 2-nitrotoluene, with about 58% being converted to the ortho- isomer, the remaining forming meta- and para- isomers.[5] The 2-nitrotoluene can then be oxidized to yield 2-nitrobenzaldehyde.[6][7]
Alternatively, 2-nitrotoluene as formed above can be halogenated to a 2-nitrobenzyl halide followed by oxidation with DMSO and sodium bicarbonate to yield 2-nitrobenzaldehyde, which is subsequently purified with the creation of a bisulfite adduct.[8]
The nitration of benzaldehyde produces mostly 3-nitrobenzaldehyde, with yields being about 19% for the ortho-, 72% for the meta- and 9% for the para isomer.[9] For this reason, the nitration of benzaldehyde to yield 2-nitrobenzaldehyde is not cost-effective.
Uses
2-Nitrobenzaldehyde is an intermediate in an early route to indigo, a water-insoluble dye commonly used to dye jeans and other fabrics. In the Baeyer-Drewson indigo synthesis, 2-nitrobenzaldehyde condenses with acetone in basic aqueous solution to yield indigo in a one-pot synthesis.[10][11][12][13] The method was abandoned in the early part of the 20th century, being replaced by routes from aniline.[14]
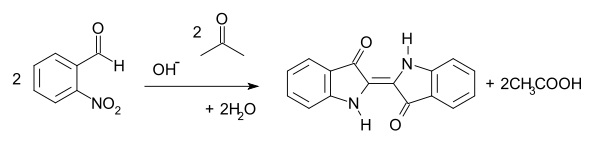 Baeyer-Drewson Indigo Synthesis
Baeyer-Drewson Indigo Synthesis
Given its two relatively reactive groups, 2-nitrobenzaldehyde is a potential starting material for other compounds. Substituted 2-nitrobenzaldehydes can also be used to yield other important compounds based on indigo, such as indigo carmine.
2-Nitrobenzaldehyde has been shown to be a useful photoremovable protecting group for various functions.[15][16]
References
- 2-Nitrobenzaldehyde
- "2-Nitrobenzaldehyde MSDS". Archived from the original on 2011-07-07. Retrieved 2009-07-18.
- o-NITROCINNAMALDEHYDE, nitration of cinnamaldehyde, organic-synthesis
- Selective aerobic oxidation of styrene to benzaldehyde catalyzed by water-soluble palladium(II) complex in water, Bo Feng, Zhenshan Hou, Xiangrui Wang, Yu Hu, Huan Li and Yunxiang Qiao
- http://www.thecatalyst.org/experiments/AndersonS/AndersonS.html Product Distribution in the Nitration of Toluene, Steven W. Anderson, January 7, 1999
- Synthesis of 2-Nitrobenzaldehyde from 2-Nitrotoluene, Alexander Popkov
- "o-Nitrobenzaldehyde". Archived from the original on 2009-07-23. Retrieved 2009-07-21.
- "Process for the Preparation of 2-Nitrobenzaldehyde". Retrieved 2010-10-18.
- Structure of Benzene, California State University Dominguez Hills
- See Baeyer-Drewson indigo synthesis
- Synthesis of Indigo Archived 2010-06-20 at the Wayback Machine
- "Indigo Synthesis". Archived from the original on 2011-07-20. Retrieved 2009-07-18.
- "Synthesis of Indigo and Vat Dyeing" (PDF). Archived from the original (PDF) on 2011-07-20. Retrieved 2009-07-18.
- Elmar Steingruber "Indigo and Indigo Colorants" Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a14_149.pub2
- Šebej, Peter; Šolomek, Tomáš; Hroudná, Ľubica; Brancová, Pavla; Klán, Petr (2009). "Photochemistry of 2-Nitrobenzylidene Acetals". J. Org. Chem. 74: 8647–8658. doi:10.1021/jo901756r.
- Kristine L. Willett; Ronald A. Hites (2000). "Chemical Actinometry: Using o-Nitrobenzaldehyde to Measure Lamp Intensity in Photochemical Experiments". J. Chem. Educ. 77: 900. doi:10.1021/ed077p900.