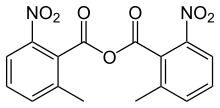
2-Methyl-6-nitrobenzoic anhydride
2-Methyl-6-nitrobenzoic anhydride is an organic acid anhydride also known as the Shiina reagent,[1][2] having a structure wherein carboxylic acids undergo intermolecular dehydration condensation. It was developed in 2002 by Prof. Isamu Shiina (Tokyo University of Science, Japan).[3] The compound is often abbreviated MNBA.
 | |
| Names | |
|---|---|
| IUPAC name
(2-Methyl-6-nitrobenzoyl) 2-methyl-6-nitrobenzoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.156.789 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12N2O7 | |
| Molar mass | 344.279 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Abstract
The reagent is used for synthetic reactions wherein medium- and large-sized lactones are formed from hydroxycarboxylic acids via intramolecular ring closure (Shiina macrolactonization).[4][5] The reaction proceeds at room temperature under basic or neutral conditions. This reagent can be used not only for macrolactonization but also for esterification, amidation, and peptide coupling.
gollark: ++apioform
gollark: ++apioform
gollark: ++apioform
gollark: ++apioform
gollark: ++apioform
See also
- Condensation reaction
- Fischer-Speier esterification
- Mitsunobu reaction
- Shiina esterification
- Steglich esterification
- Yamaguchi esterification
References
- "Named Reagents". OChemOnline. Archived from the original on 2017-09-04.
- Yahata, K.; Ye, N.; Iso, K.; Naini, S. R.; Yamashita, S.; Ai, Y.; Kishi, Y. (2017). "Unified Synthesis of Right Halves of Halichondrins A–C". J. Org. Chem. 82 (17): 8792–8807. doi:10.1021/acs.joc.7b01283. PMID 28741352.
- Shiina, I.; Ibuka, R.; Kubota, M. (2002). "A New Condensation Reaction for the Synthesis of Carboxylic Esters from Nearly Equimolar Amounts of Carboxylic Acids and Alcohols Using 2-Methyl-6-nitrobenzoic Anhydride". Chem. Lett. 31 (3): 286. doi:10.1246/cl.2002.286.
- Shiina, I.; Kubota, M.; Oshiumi, H.; Hashizume, M. (2004). "An Effective Use of Benzoic Anhydride and Its Derivatives for the Synthesis of Carboxylic Esters and Lactones: A Powerful and Convenient Mixed Anhydride Method Promoted by Basic Catalysts". J. Org. Chem. 69 (6): 1822–30. doi:10.1021/jo030367x. PMID 15058924.
- Shiina, I. (2014). "An Adventurous Synthetic Journey with MNBA from Its Reaction Chemistry to the Total Synthesis of Natural Products". Bull. Chem. Soc. Jpn. 87 (2): 196–233. doi:10.1246/bcsj.20130216.
External links
- 2-Methyl-6-nitrobenzoic Anhydride (MNBA)
- Enantioselective Total Synthesis of Octalactin A Using Asymmetric Aldol Reactions and a Rapid Lactonization To Form a Medium-Sized Ring
- Total Synthesis of Iejimalide B. An Application of the Shiina Macrolactonization
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.