2-Aminobiphenyl
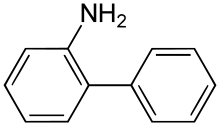
2-Aminobiphenyl (2-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored even black.[1] Palladacycles obtained from 2-aminobiphenyl are popular catalysts for cross-coupling.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
[1,1'-Biphenyl]-2-amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.810 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H11N | |
| Molar mass | 169.227 g·mol−1 |
| Appearance | white solid |
| Density | 1.077 g/cm3 |
| Melting point | 51 °C (124 °F; 324 K) |
| Boiling point | 299 °C (570 °F; 572 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H351, H412 |
| P201, P202, P264, P270, P273, P281, P301+312, P308+313, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is prepared by hydrogenation of 2-nitrobiphenyl.[3]
See also
References
- M. J. S. Dewar; R. B. K. Dewar; Z. L. F. Gaibel (1966). "10-Methyl-10,9-borazarophenanthrene". Org. Synth. 46: 65. doi:10.15227/orgsyn.046.0065.
- Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. (2015). "2-Aminobiphenyl Palladacycles: The "Most Powerful" Precatalysts in C–C and C–Heteroatom Cross-Couplings". ACS Catalysis. 5: 1386-1396. doi:10.1021/cs502011x.
- G. David Mendenhall; Peter A. S. Smith (1966). "2-Nitrocarbazole". Org. Synth. 46: 85. doi:10.15227/orgsyn.046.0085.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.