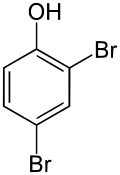
2,4-Dibromophenol
2,4-Dibromophenol is a brominated derivative of phenol with the molecular formula C6H4Br2O. It belongs to the bromobenzenes, which are organic compounds containing bromine atoms attached to a benzene ring.
 | |
| Names | |
|---|---|
| IUPAC name
2,4-Dibromophenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.488 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H4Br2O | |
| Molar mass | 251.905 g·mol−1 |
| Melting point | 38 °C (100 °F; 311 K) |
| Boiling point | 238.5 °C (461.3 °F; 511.6 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H300, H315, H319, H335, H412 |
| P261, P264, P270, P271, P273, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
At room temperature, 2,4-dibromophenol is a solid with needle-like crystals. It melts at 38 °C (100.4 °F) and boils at 238.5 °C (461.3 °F). it has a molecular weight of 251.905 g/mol. It is soluble in water, ethanol, ether and benzene and slightly soluble in carbon tetrachloride.[1]
Occurrence
2,4-Dibromophenol is found in certain molluscs and crustaceans,[1] as well as the acorn worm Saccoglossus bromophenolosus, which is named after it.[2]
gollark: Also, did you consider websockets instead of some weird HTTP polling?
gollark: <@426660245738356738> Can you share the code for the HTTP server this presumably connects to?
gollark: Still, I think that trying to use CC without programming anything... kind of defeats the point of using CC.
gollark: Well, that seems somewhat more useful than other OSes I guess?
gollark: It's not tied into THOR though.
References
- CID 12005 from PubChem
- Konikoff, Charlotte (2017). "Saccoglossus bromophenolosus King, Giray & Kornfield, 1994". WoRMS. World Register of Marine Species. Retrieved 31 August 2017.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.