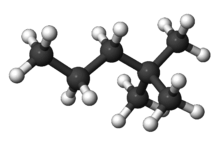
2,2-Dimethylpentane
2,2-Dimethylpentane is one of the isomers of heptane. It is also called neoheptane as it contains the (CH3)3C grouping. It has the most extreme properties of the isomers.
 | |
| Names | |
|---|---|
IUPAC name
| |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1730757 | |
| ChemSpider | |
| ECHA InfoCard | 100.008.801 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H16 | |
| Molar mass | 100.205 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.67385 g cm−3 (liquid, 20 °C, 1 atm) 0.6695 g cm−3 (liquid, 25 °C, 1 atm)[1] |
| Melting point | −123.7 °C; −190.8 °F; 149.4 K |
| Boiling point | 79.2 °C; 174.5 °F; 352.3 K |
| Structure | |
| 0.0 D | |
| Thermochemistry | |
Heat capacity (C) |
221.12 J K−1 mol−1[2] |
Std molar entropy (S |
300.3 J K−1 mol−1[2] |
Std enthalpy of formation (ΔfH⦵298) |
-238.5 kJ mol−1[2] |
Std enthalpy of combustion (ΔcH⦵298) |
-4802.94 kJ mol−1[2] |
| Hazards[3] | |
| Safety data sheet | [4] |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H304, H315, H335, H336, H400, H410 |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+310, P302+352, P303+361+353, P304+340, P312, P321, P331, P332+313, P362, P370+378, P391, P403+233, P403+235, P405 | |
| Flash point | 15[5] °C (59 °F; 288 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
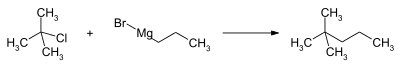
Preparation
A method to produce high purity 2,2-dimethylpentane is to react the grignard reagent of n-propyl bromide (CH3CH2CH2MgBr) with tert-butyl chloride ((CH3)3CCl).[6] n-PrMgCl can be used instead of the bromide.[7]
Properties
In 1929 Graham Edgar and George Calingaert made 2,2-dimethylpentane and measured its physical characteristics for the first time. The measurements were at 20°C, not the standard conditions used in later times.[8]
For 2,2-dimethylpentane they measured a density of 0.6737 at 20°C with a rate of change Δd/ΔT of 0.000855. The density and boiling are the lowest of the isomers of heptane. The dielectric constant is 1.915, the lowest of the heptane isomers. The critical temperature is 247.7°C and critical pressure 28.4 atmospheres. The refractive index at 20° is 1.38233, the same as for 2,4-dimethylpentane, equal lowest for the heptane isomers. The adiabatic compressibility is 0.0001289 and isothermal compressibility is 0.00016279 atmospheres, highest for the heptanes. The velocity of sound is 1.080 km/s which is lowest of the heptanes. Coefficient of thermal expansion is 0.001268/°, highest of the heptanes. Surface tension is 17.80 dynes/cm. Viscosity is 0.00385. The heat of combustion is 11470 cal/g which is very similar to other heptanes.[8]
Reactions
2,2-Dimethylpentane can form a clathrate hydrate with helper gas molecules. The type of clathrate formed is called "clathrate H". 2,2-Dimethylpentane was the first compound for which the structure was determined. The clathrate has 34 molecules of water per molecule, and also has xenon and hydrogen sulfide as helper molecules. The crystals are hexagonal in form and melt at 0.6°C. This substance is important as clathrate hydrates form problems by blocking natural gas pipelines.[9]
2,2-Dimethylpentane does not react with bromine, iodine, nitric acid or chlorosulfonic acid because there are no tertiary carbon atoms (a carbon atom with only one hydrogen attached).[10]
Heating alkanes over an aluminium trichloride is used to reform to make different isomers. 2,2-Dimethylpentane does not participate in this process and so cannot be reformed, or created by reforming other heptanes.[11][12]
Natural occurrence
2,2-Dimethylpentane exists in some crude oils at low levels[10] of about 0.01%.[13]
References
- 马沛生. 有机化合物实验物性数据手册--含碳氢氧卤部分[M]. 化学工业出版社, 2006:170.
- 马沛生. 有机化合物实验物性数据手册--含碳氢氧卤部分[M]. 化学工业出版社, 2006:466.
- "2,2-Dimethylpentane". pubchem.ncbi.nlm.nih.gov.
- "Material Safety Data Sheet 2,2-Dimethylpentane, 99+%". fscimage.fishersci.com.
- 戈克尔. 有机化学手册[M]. 化学工业出版社, 2006:174.
- Edgar, Graham; Calingaert, George; Marker, R. E. (May 1929). "The Preparation And Properties Of The Isomeric Heptanes. Part I. Preparation". Journal of the American Chemical Society. 51 (5): 1483–1491. doi:10.1021/ja01380a027.
- Soroos, Harold; Willis, H. B. (March 1941). "The Preparation of 2,2- and 3,3-Dimethylpentane". Journal of the American Chemical Society. 63 (3): 881. doi:10.1021/ja01848a515.
- Edgar, Graham; Calingaert, George (May 1929). "The Preparation And Properties Of The Isomeric Heptanes. Part II. Physical Properties". Journal of the American Chemical Society. 51 (5): 1540–1550. doi:10.1021/ja01380a035.
- Udachin, K. A.; Ratcliffe, C. I.; Enright, G. D.; Ripmeester, J. A. (May 1997). "Structure H Hydrate: A Single Crystal Diffraction Study of 2,2-dimethylpentane·5(Xe, H2S)·34H2O". Supramolecular Chemistry. 8 (3): 173–176. doi:10.1080/10610279708034933.
- Bruun, J.H.; Bruun, M.M.H. (July 1932). "Note on the probable presence of 2,2-dimethylpentane in a midcontinent petroleum". Bureau of Standards Journal of Research. 9 (1): 53. doi:10.6028/jres.009.008.
- van Eijk van Voorthuijsen, J. J. B. (3 September 2010). "The isomerization equilibria of the butanes, pentanes, hexanes and heptanes". Recueil des Travaux Chimiques des Pays-Bas. 66 (5): 323–334. doi:10.1002/recl.19470660508.
- Anderson, Hazel C. (1954). Bibliography of the Fischer-Tropsch Synthesis and Related Processes: Review and compilation of the literature on the production of synthetic liquid fuels and chemicals by the hydrogenation of carbon monoxide. U.S. Government Printing Office. p. 462.
- Atarshchikov, S. V.; Mirimanyan, A. A.; Mkrtychev, A. A. (September 2005). "Moderate-Temperature Isomerizate — A High-Octane Component of Automotive Gasoline". Chemistry and Technology of Fuels and Oils. 41 (5): 362–369. doi:10.1007/s10553-005-0081-9.