2,2'-Dipyridylamine
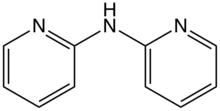
2,2'-Dipyridylamine is an organic compound with the formula (C5H4N)2NH. It consists of a pair of 2-pyridyl groups (C5H4N) linked to a secondary amine. The compound forms a range of coordination complexes.[1] Its conjugate base, 2,2'-dipyridylamide, forms extended metal atom chains.[2]
 | |
| Names | |
|---|---|
| IUPAC name
N-pyridin-2-ylpyridin-2-amine | |
| Other names
2,2'-iminodipyridine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.013.513 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H9N3 | |
| Molar mass | 171.203 g·mol−1 |
| Appearance | white solid |
| Melting point | 90.5 °C (194.9 °F; 363.6 K) |
| Boiling point | 307.5 °C (585.5 °F; 580.6 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Formation
2,2'-Dipyridylamine can be formed by heating pyridine with sodium amide. Alternatively, 2-aminopyridine can be heated with 2-chloropyridine over barium oxide.[3]
gollark: Interesting.
gollark: You can just use any vaguely recognisable image as a profile picture like I and indeed many other people here do, it doesn't have to contain faces.
gollark: https://pbs.twimg.com/media/EkM8L3IXsAUaBNw?format=jpg
gollark: > consuming pizza
gollark: It's very memetic.
References
- Wang, Suning (2001). "Luminescence and electroluminescence of Al(III), B(III), Be(II) and Zn(II) complexes with nitrogen donors". Coordination Chemistry Reviews. 215: 79–98. doi:10.1016/S0010-8545(00)00403-3.
- Cotton, F. Albert; Daniels, Lee M.; Jordan; Murillo, Carlos A. (1997). "Symmetrical and Unsymmetrical Compounds Having a Linear Co36+ Chain Ligated by a Spiral Set of Dipyridyl Anions". Journal of the American Chemical Society. 119 (43): 10377–10381. doi:10.1021/JA971997H.
- Brogden, David W.; Berry, John F. (4 September 2015). "Coordination Chemistry of 2,2'-Dipyridylamine: The Gift That Keeps on Giving". Comments on Inorganic Chemistry. 36 (1): 17–37. doi:10.1080/02603594.2015.1079522.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.