2,2'-Biquinoline
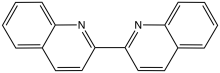
2,2'-Biquinoline is an organic compound with the formula (C9H6N)2. It is one of several dimers of the bicyclic heterocycle called quinoline. It is prepared by reductive coupling of 2-chloroquinoline.[1] It is a colorimetric indicator for organolithium compounds.[2]
 | |
| Names | |
|---|---|
| Other names
2,2'-Biquinolyl; 2-Quinolin-2-ylquinoline; Cuproin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.961 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H12N2 | |
| Molar mass | 256.308 g·mol−1 |
| Appearance | White solid |
| Melting point | 194.5 °C (382.1 °F; 467.6 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Nelson, Todd D.; Crouch, R. David (2004). "Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction". Org. Reactions. 15. doi:10.1002/0471264180.or063.03.
- Watson, Spencer Charles; Eastham, Jerome F. (1967). "Colored Indicators for Simple Direct Titration of Magnesium and Lithium Reagents". Journal of Organometallic Chemistry. 9: 165-8. doi:10.1016/S0022-328X(00)92418-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.