2,2'-Biphenylene phosphorochloridite
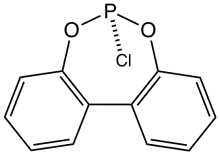
2,2'-Biphenylene phosphorochloridite is the name for a polycyclic organophosphorus compound with the formula C12H8O2PCl. It is a precursor to diphosphite ligands such as BiPhePhos by reaction with suitable diols. 2,2'-Biphenylene phosphorochloridites, which is a white solid, is prepared from 2,2'-biphenol and phosphorus trichloride. It is prepared by the reaction of 2,2'-biphenol and phosphorus trichloride.[1][2]
 | |
| Names | |
|---|---|
| IUPAC name
6-Chlorodibenzo[d,f][1,3,2]dioxaphosphepine | |
| Other names
1,1’-biphenyl-2,2’-diyl phosphorochloridite | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H8ClO0P | |
| Molar mass | 218.62 g·mol−1 |
| Appearance | white solid |
| Melting point | 63 °C (145 °F; 336 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized α-Olefins". Journal of the American Chemical Society. 115: 2066–2068. doi:10.1021/ja00058a079.
- Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics. 15: 835–847. doi:10.1021/OM950549K.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.