18F-EF5
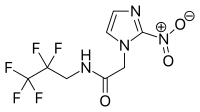
EF5 is a nitroimidazole derivative used in oncology research.[1] Due to its similarity in chemical structure to etanidazole, EF5 binds in cells displaying hypoxia.[2]
 | |
| Names | |
|---|---|
| IUPAC name
2-(2-Nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide | |
| Other names
NSC-684681; EF-5 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H7F5N4O3 | |
| Molar mass | 302.161 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The 18F-radiolabeled derivative of EF5 is being studied for its possibility to be used in positron emission tomography (PET) to detect low levels of oxygen in brain tumors.[3] This can help show how a tumor will respond to treatment.
References
- Evans, Sydney M.; Fraker, Douglas; Hahn, Stephen M.; Gleason, Kristen; Jenkins, W. Timothy; Jenkins, Kevin; Hwang, Wei-Ting; Zhang, Paul; Mick, Rosemarie; Koch, Cameron J (2006). "EF5 binding and clinical outcome in human soft tissue sarcomas". International Journal of Radiation Oncology, Biology, Physics. 64 (3): 922–927. doi:10.1016/j.ijrobp.2005.05.068.
- Lord, Edith M.; Harwell, Lee; Koch, Cameron J. (1993). "Detection of hypoxic cells by monoclonal antibody recognizing 2-nitroimidazole adducts". Cancer Research. 53 (23): 5721–5726. PMID 8242628.
- Ziemer, L. S.; Evans, S. M.; Kachur, A. V.; Shuman, A. L.; Cardi, C. A.; Jenkins, W. T.; Karp, J. S.; Alavi, A.; Dolbier, W. R.; Koch, C. J. (2003). "Noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18F-EF5". European Journal of Nuclear Medicine and Molecular Imaging. 30 (2): 259–266. doi:10.1007/s00259-002-1037-5. PMID 12552344.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.