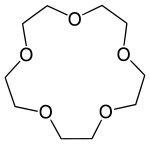
15-Crown-5
15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+)[2] and potassium (K+),[3] however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
1,4,7,10,13-Pentaoxacyclopentadecane[1] | |
| Identifiers | |
3D model (JSmol) |
|
| 1618144 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.046.694 |
| EC Number |
|
| 3897 | |
| MeSH | 15-Crown-5 |
PubChem CID |
|
| RTECS number |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H20O5 | |
| Molar mass | 220.265 g·mol−1 |
| Appearance | Clear, colorless liquid |
| Density | 1.113 g cm−3 (at 20 °C) |
| Boiling point | 93–96 °C (199–205 °F; 366–369 K) at 0.05 mmHg |
| log P | -0.639 |
Refractive index (nD) |
1.465 |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-881.1--877.1 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
-5.9157--5.9129 MJ mol−1 |
| Hazards | |
| Safety data sheet | msds.chem.ox.ac.uk |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H315, H319 |
| P305+351+338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 113 °C (235 °F; 386 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Analogous to 18-crown-6, 15-crown-5 binds to sodium ions. Thus, when treated with this complexing agent, sodium salts often become soluble in organic solvents.
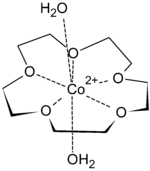
First-row transition metal dications fit snugly inside the cavity of 15-crown-5. They are too small to be included in 18-crown-6. The binding of transition metal cations results in multiple hydrogen-bonded interactions from both equatorial and axial aqua ligands, such that highly crystalline solid-state supramolecular polymers can be isolated. Metal salts isolated in this form include Co(ClO4)2, Ni(ClO4)2, Cu(ClO4)2, and Zn(ClO4)2. Seven coordinate species are most common for transition metal ions complexes of 15-crown-5, with the crown ether occupying the equatorial plane, along with 2 axial aqua ligands.[4]
15-crown-5 has also been used to isolate salts of oxonium ions. For example, from a solution of tetrachloroauric acid, the oxonium ion [H7O3]+ has been isolated as the salt [(H7O3)(15-crown-5)2][AuCl4]. Neutron diffraction studies revealed a sandwich structure, which shows a chain of water with remarkably long O-H bond (1.12 Å) in the acidic proton, but with a very short OH•••O distance (1.32 Å).[4]
(15-crown-5))%2B.jpg)
A derivative of 15-crown-5, benzo-15-crown-5, has been used to produce anionic complexes of carbido ligands as their [K(benzo-15-crown-5)2]+ salts:[4]
- (Ar2N)3MoCH + KCH2Ph + 2 (15-crown-5) → [K(15-crown-5)2]+[(Ar2N)3MoC]− + CH3Ph
See also
- Host guest chemistry
- Phase transfer catalyst
References
- "15-crown-5 - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 11 October 2011.
- Takeda, Y.; et al. (1988). "A Conductance Study of 1:1 Complexes of 15-Crown-5, 16-Crown-5, and Benzo-15-crown-5 with Alkali Metal Ions in Nonaqueous Solvents". Bulletin of the Chemical Society of Japan. 61 (3): 627–632. doi:10.1246/bcsj.61.627.
- Chen, Chun-Yen; et al. (2006). "Potassium ion recognition by 15-crown-5 functionalized CdSe/ZnS quantum dots in H2O". Chem. Comm. (3): 263–265. doi:10.1039/B512677K.
- Jonathan W. Steed; Jerry L. Atwood (2009). Supramolecular Chemistry, 2nd edition. Wiley. ISBN 978-0-470-51233-3.
Further reading
- Klok, H.A.; et al. (1997). "Novel benzo-15-crown-5 functionalized α-olefin/CO terpolymers for membrane applications". Macromolecular Chemistry and Physics. 198 (9): 2759–2768. doi:10.1002/macp.1997.021980908.
- Fedorova, O.A.; et al. (2005). "Facile synthesis of novel styryl ligands containing a 15-crown-5 ether moiety". Arkivoc. xv: 12–24.
