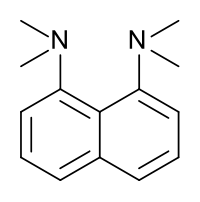
1,8-Bis(dimethylamino)naphthalene
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N1,N1,N8,N8-tetramethylnaphthalene-1,8-diamine | |
| Other names
N,N,N',N'-tetramethylnaphthalene-1,8-diamine Proton Sponge | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.039.986 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H18N2 | |
| Molar mass | 214.312 g·mol−1 |
| Appearance | White crystaline powder |
| Melting point | 47.8 °C (118.0 °F; 320.9 K) |
| Acidity (pKa) | 12.1 (in water)[1] 18.62 (in acetonitrile)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and properties
This compound is a diamine in which the two dimethylamino groups are attached on the same side (peri position) of a naphthalene ring. This molecule has several very interesting properties; one is its very high basicity; another is its spectroscopic properties.
With a pKa of 12.34 [4] for its conjugate acid in aqueous solution, 1,8-bis(dimethylamino)naphthalene is one of the strongest organic bases. However, it only absorbs protons slowly—hence the trade name. The high basicity is attributed to the relief of strain upon protonation and/or the strong interaction between the nitrogen lone pairs.[3] Additionally, although many aromatic amines such as aniline show reduced basicity (due to nitrogen being sp² hybridized; its lone pair occupying a 2p orbital and interacting and being withdrawn by the aromatic ring), this is not possible in this molecule, as the nitrogens' methyl groups prevent its substituents from adopting a planar geometry, as this would require forcing methyl groups from each nitrogen atom into one another - thus the basicity is not reduced by this factor which is found in other molecules. It is sterically hindered, making it a weak nucleophile. Because of this combination of properties, it has been used in organic synthesis as a highly selective non-nucleophilic base.[4]
Proton sponge also exhibits a very high affinity for boron, and is capable of displacing hydride from borane to form a boronium–borohydride ion pair.[5]
Preparation
This compound is commercially available. It may be prepared by the methylation of 1,8-diaminonaphthalene with iodomethane or dimethyl sulfate.[6]
Related compounds
Other proton sponges
Second generation proton sponges are known with even higher basicity. 1,8-bis(hexamethyltriaminophosphazenyl)naphthalene or HMPN[7] is prepared from 1,8-diaminonaphthalene by reaction with tris(dimethylamino)bromophosphonium bromide in the presence of triethylamine. HMPN has a pKBH+ of 29.9 in acetonitrile which is more than 11 orders of magnitude higher than Proton Sponge.
Hydride sponge
The chemical inverse of a proton sponge would be a hydride sponge. This property is exhibited by C10H6(BMe2)2, which reacts with potassium hydride to afford K[C10H6(BMe2)2H].[8]
References
- R. W. Alder; P. S. Bowman; W. R. S. Steele & D. R. Winterman (1968). "The remarkable basicity of 1,8-bis(dimethylamino)naphthalene". Chem. Commun. (13): 723. doi:10.1039/C19680000723.
- I. Kaljurand, A. Kütt, L. Sooväli, T. Rodima, V. Mäemets, I. Leito, I. A. Koppel. Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales. J. Org. Chem., 2005, 70, 1019–1028. doi:10.1021/jo048252w
- R. W. Alder (1989). "Strain effects on amine basicities". Chem. Rev. 89 (5): 1215–1223. doi:10.1021/cr00095a015.
- Alexander F. Pozharskii and Valery A. Ozeryanskii "Proton sponges and hydrogen transfer phenomena" Mendeleev Commun., 2012, 22, 117–124. doi:10.1016/j.mencom.2012.05.001
- Légaré, Marc-André; Courtemanche, Marc-André; Fontaine, Frédéric-Georges (2014-08-28). "Lewis base activation of borane–dimethylsulfide into strongly reducing ion pairs for the transformation of carbon dioxide to methoxyboranes". Chemical Communications. 50 (77). doi:10.1039/c4cc04857a. hdl:20.500.11794/29769. ISSN 1364-548X.
- Sorokin, Vladimir I.; Ozeryanskii, Valery A.; Pozharskii, Alexander F. (2003). "A Simple and Effective Procedure for the N-Permethylation of Amino-Substituted Naphthalenes". European Journal of Organic Chemistry. 2003 (3): 496. doi:10.1002/ejoc.200390085.
- Volker Raab; Ekaterina Gauchenova; Alexei Merkoulov; Klaus Harms; Jörg Sundermeyer; Borislav Kovačević & Zvonimir B. Maksić (2005). "1,8-Bis(hexamethyltriaminophosphazenyl)naphthalene, HMPN: A Superbasic Bisphosphazene "Proton Sponge"". J. Am. Chem. Soc. 127 (45): 15738–15743. doi:10.1021/ja052647v. PMID 16277515.
- Katz, Howard Edan (1985). "Hydride sponge: 1,8-naphtalenediylbis(dimethylborane)". Journal of the American Chemical Society. 107 (5): 1420–1421. doi:10.1021/ja00291a057.