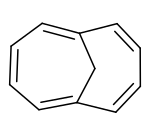
1,6-Methano(10)annulene
1,6-Methano[10]annulene (also known as 1,6-methanonaphthalene or homonaphthalene) is an aromatic hydrocarbon with chemical formula C11H10. It was the first stable aromatic compound based on the cyclodecapentaene system to be discovered.
 | |
| Names | |
|---|---|
| IUPAC name
Bicyclo[4.4.1]undeca-1,3,5,7,9-pentaene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H10 | |
| Molar mass | 142.201 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
According to Organic Syntheses, it can be prepared from naphthalene.[1]
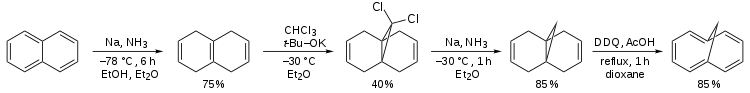 Synthetic route to 1,6-methano[10]annulene
Synthetic route to 1,6-methano[10]annulene
Aromaticity
It is analogous to cyclodecapentaene ([10]annulene), but with two hydrogen atoms replaced by a transannular methylene bridge (-CH
2-). Consequently, it obeys Hückel's rule (n = 2) and despite the distortion from planarity introduced by the methylene bridge, the compound is aromatic.[2][3] In fact, when prepared by Vogel in the 1960s[4][5] it was the first stable aromatic cyclodecapentaene to be discovered.[3] [Note: It was later discovered that in actuality, this molecule was first prepared by Heinz D. Roth, who showed the molecule to Vogel, the man who consequently took credit for the work.] Its aromaticity is confirmed by three main pieces of evidence. Firstly, the similarity in carbon-carbon bond lengths as measured by x-ray crystallography is inconsistent with alternating single and double bonds. The actual structure is better considered as a pair of resonance hybrids (like the Kekulé structures of benzene) rather than as having alternating single and double bonds.
Secondly, its 1H NMR spectrum displays influence of the diamagnetic ring current which is characteristic of aromatic compounds. The peripheral protons around the ring are deshielded while the methylene bridge nuclei are strongly shielded.[2][3]
Its resonance energy is smaller than that of naphthalene.[6]
References
- Vogel, Emanuel; Klug, W.; Breuer, A. "1,6-Methano[10]annulene". Organic Syntheses. 54: 11. doi:10.15227/orgsyn.054.0011.; Collective Volume, 6, p. 731
- Gatti, Carlo; Orlando, Ahmed M.; Monza, Emanuele; Lo Presti, Leonardo (2016). "Exploring Chemistry Through the Source Function for the Electron and the Electron Spin Densities". In Chauvin, Remi; Lepetit, Christine; Silvi, Bernard; Alikhani, Esmail (eds.). Applications of Topological Methods in Molecular Chemistry. Challenges and Advances in Computational Chemistry and Physics. 22. Springer International Publishing. pp. 101–129. doi:10.1007/978-3-319-29022-5_5. ISBN 9783319290225.
- Hill, Richard K.; Giberson, Carolyn B.; Silverton, James V. (1988). "Forfeiture of the aromaticity of a bridged [10]annulene by benzannelation". J. Am. Chem. Soc. 110 (2): 497–500. doi:10.1021/ja00210a031.
- Vogel, Emanuel; Roth, H. D. (1964). "The Cyclodecapentaene System". Angew. Chem. Int. Ed. 3 (3): 228–229. doi:10.1002/anie.196402282.
- Vogel, Emanuel; Böll, W. A. (1964). "Substitution of 1,6-Methanocyclodecapentaene". Angew. Chem. Int. Ed. 3 (9): 642. doi:10.1002/anie.196406421.
- Roth, Wolfgang R.; Böhm, Manfred (1983). "Resonance Energy of Bridged [10]Annulene". Angew. Chem. Int. Ed. 22 (12): 1007–1008. doi:10.1002/anie.198310071.