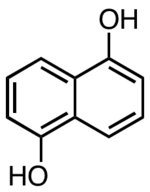
1,5-Dihydroxynaphthalene
1,5-Dihydroxynaphthalene is an organic compound with the formula C10H6(OH)2. It is one of several isomers of dihydroxynaphthalene. It exists as colorless crystals that are soluble in polar organic solvents. It is a precursor to certain dyes.
 | |
| Names | |
|---|---|
| IUPAC name
Naphthalene-1,5-diol | |
| Other names
Azurol; 1,5-Naphthalenediol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.353 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H8O2 | |
| Molar mass | 160.172 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 259–261 °C (498–502 °F; 532–534 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and use
1,5-Dihydroxynaphthalene is prepared from naphthalene-1,5-disulfonic acid by hydrolysis with strong base followed by acidification.
It couples with various aryl diazonium salts to give diazo dyes. Oxidation with chromium trioxide gives juglone, a naturally occurring dye.[1]
gollark: I disagree.
gollark: Imagine having legs.
gollark: That sure is a band of some kind?
gollark: I disagree with this.
gollark: Necessarily, under anarchoprimitivism.
References
- Gerald Booth (2005). "Naphthalene Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_009..
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.