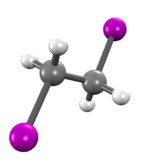
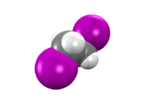
1,2-Diiodoethane
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2-diiodoethane | |||
| Other names
ethylene iodide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.009.872 | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4I2 | |||
| Molar mass | 281.863 g·mol−1 | ||
| Density | 2.13 g/cm3 | ||
| Melting point | 80 to 82 °C (176 to 180 °F; 353 to 355 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,2-Diiodoethane is an organoiodine compound.[1]
Preparation and reactions
1,2-Diiodoethane can be prepared by the reaction of ethylene with iodine (I2):[2]
- C2H4 + I2 ⇌ C2H4I2
1,2-Diiodoethane is most commonly used in organic synthesis in the preparation of samarium(II) iodide or ytterbium(II) iodide in an inert solvent such as THF.[3]
- Sm + ICH2CH2I → SmI2 + H2C=CH2
Spectral properties
In mass spectroscopy, 1,2-diiodoethane exhibits 5 major peaks, with the base peak showing at 155 m/z, which is the loss of one iodine atom (127 m/z).
gollark: There are new innovations in nuclear power which could improve efficiency, reduce cost and improve safety too, except nobody seems to be implementing them because people seem to just... not like nuclear.
gollark: Nuclear waste isn't actually a huge issue - you could fit all nuclear waste generated so far into a small swimming pool or something and it's *much* better than the effects of fossil fuel pollution - and meltdowns are rare.
gollark: batery™ is expense™ and bad compared to not needing batery™.
gollark: Nuclear power: EXTREMELY COOL.
gollark: Wind turbines: UNCOOL.
References
- Buckingham, John (1990). Dictionary of Organic Compounds. 7. CRC Press. p. 2495. ISBN 9780412540905. Retrieved 5 January 2014.
- Cutherbertson, G.R.; Kistiakowsky, G.B. (1935). "The thermal equilibrium between ethylene iodide, ethylene and iodine". J. Chem. Phys. 3 (10): 631–634. Bibcode:1935JChPh...3..631C. doi:10.1063/1.1749566.
- Girard, P.; Namy, J. L.; Kagan, H. B. (1980). "Divalent lanthanide derivatives in organic synthesis. 1. Mild preparation of samarium iodide and ytterbium iodide and their use as reducing or coupling agents". Journal of the American Chemical Society. 102 (8): 2693–2698. doi:10.1021/ja00528a029.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.