1,2-Dihydro-1,2-azaborine
1,2-Dihydro-1,2-azaborine is an aromatic chemical compound with properties intermediate between benzene and borazine. Its chemical formula is C4BNH6. It resembles a benzene ring, except that two adjacent carbons are replaced by nitrogen and boron, respectively.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
| ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H6BN | |||
| Molar mass | 78.908 g mol−1 | ||
| Appearance | clear, colorless liquid | ||
| Melting point | −46 to −45 °C. | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
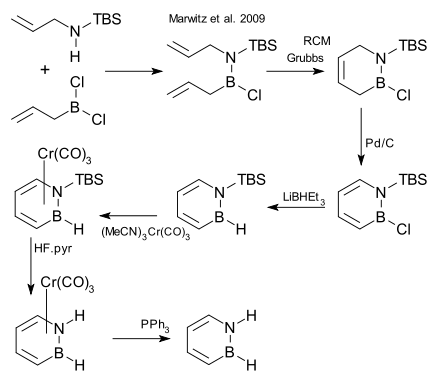
After decades of failed attempts, the compound was synthesized in 2008 and reported in January 2009.[1][2]
One of the synthetic steps is a ring-closing metathesis (RCM) reaction:[3]
gollark: Mere sort?
gollark: Too bad, you will be rotated in 7-space.
gollark: Just get good and read the machine code.
gollark: Well, I find your dichotomy "literal apioform".
gollark: That's reverse engineering of things, you literal metaphorical bee.
References
- Stu Borman. "Long-Sought Benzenelike Molecule Created: Aromaticity of organic-inorganic hybrid resembles benzene's." C&EN January 5, 2009 Volume 87, Number 01 p. 11
- A. J. V. Marwitz; M. H. Matus; L. N. Zakharov; D. A. Dixon; S.-Y. Liu (January 2009). "A Hybrid Organic/Inorganic Benzene". Angew. Chem. Int. Ed. 48 (5): 973–977. doi:10.1002/anie.200805554. PMID 19105174.
- TBS = tert-butyldimethylsilyl, step 2 RCM = ring-closing metathesis using Grubbs' catalyst, step 3 organic oxidation using palladium on carbon, step 4 reduction LiBHEt3, step 5 conversion to piano stool complex as protective group with chromium carbonyl derivative, step 6 cleavage N-TBS bond HF, step 7 deprotection with triphenylphosphine
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.