1,2-Dichloro-1,1,2-trifluoroethane
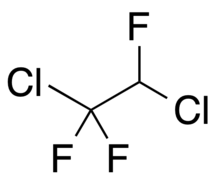
1,2-Dichloro-1,1,2-trifluoroethane is a volatile liquid chlorofluoroalkane composed of carbon, hydrogen, chlorine and fluorine, and with structural formula CClF2CHClF. It is also known as a refrigerant with the designation R-123a.[1]
 | |
| Names | |
|---|---|
| IUPAC name
1,2-dichloro-1,1,2-trifluoroethane | |
| Other names
R-123a | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3163 1078 |
| |
| |
| Properties | |
| C2HCl2F3 | |
| Molar mass | 152.93 g·mol−1 |
| Density | 1.50 |
| Melting point | −78.0 °C (−108.4 °F; 195.2 K) |
| Boiling point | 29.5 °C (85.1 °F; 302.6 K) |
| Vapor pressure | 620.01 mmHg |
Henry's law constant (kH) |
9.55×10-2 atm-cu m/mole |
Refractive index (nD) |
1.327 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Formation
1,1,2-Trichloro-1,2,2-trifluoroethane can be biotransformed in sewage sludge to 1,2-dichloro-1,1,2-trifluoroethane.[2]
Properties
The critical temperature of R-123a is 461.6 K (188.5 °C; 371.2 °F).[3] The rotation of the molecule appears to be hindered by the present of chlorine on each carbon atom, but is eased at higher temperatures.[3]
Use
Although not deliberately used, R-123a is a significant impurity in its isomer, the widely used 2,2-dichloro-1,1,1-trifluoroethane (R-123).[3]
gollark: I guess so... why?
gollark: Right, so just sign in with your license key or whatever on the remote endpoint.
gollark: I think you would need to set up the license key or something, but yes.
gollark: Ale has a JS module for it.
gollark: It's meant to mostly support the old API.
References
- Kubota, H.; Yamashita, T.; Tanaka, Y.; Makita, T. (May 1989). "Vapor pressures of new fluorocarbons". International Journal of Thermophysics. 10 (3): 629–637. doi:10.1007/BF00507984.
- Christian Balsiger; David Werner; Christof Holliger; Patrick Höhener (10 January 2005). "Reductive dechlorination of CFCs and HCFCs under methanogenic conditions" (PDF). Retrieved 15 July 2020.
- Goodwin, A. R. H.; Moldover, M. R. (October 1991). "Thermophysical properties of gaseous refrigerants from speed‐of‐sound measurements. III. Results for 1,1‐dichloro‐2,2,2‐trifluoroethane (CHCl2‐CF3) and 1,2‐dichloro‐1,2,2‐trifluoroethane (CHClF‐CClF2)". The Journal of Chemical Physics. 95 (7): 5236–5242. doi:10.1063/1.461831.
External links
- OKADA, Masaaki (2 December 1995). "代替冷媒の表面張力" [Surface Tension of Alternative Refrigerants]. Trans. of the JAR (in Japanese). 12 (2): 135–145. doi:10.11322/tjsrae.12.135.

This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.