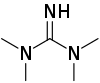
1,1,3,3-Tetramethylguanidine
Tetramethylguanidine is an organic compound with the formula HNC(N(CH3)2)2. This colourless liquid is a strong base, as judged by the high pKa of it conjugate acid.[2]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1,3,3-Tetramethylguanidine | |
| Identifiers | |
3D model (JSmol) |
|
| 969608 | |
| ChemSpider | |
| ECHA InfoCard | 100.001.185 |
| EC Number |
|
| MeSH | 1,1,3,3-tetramethylguanidine |
PubChem CID |
|
| UNII | |
| UN number | 2920 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H13N3 | |
| Molar mass | 115.180 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 918 mg mL−1 |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 160 to 162 °C (320 to 324 °F; 433 to 435 K) |
| Miscible | |
| Vapor pressure | 30 Pa (at 20 °C) |
| Acidity (pKa) | 13.0±1.0[1] (pKa of conjugate acid in water) |
Refractive index (nD) |
1.469 |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H302, H314 |
| P280, P305+351+338, P310 | |
| Flash point | 60 °C (140 °F; 333 K) |
| Explosive limits | 1–7.5% |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It was originally prepared from tetramethylthiourea via S-methylation and amination, but alternative methods start from cyanogen iodide.[3]
Uses
TMG is mainly used as a strong, non-nucleophilic base for alkylations, often as a substitute for the more expensive DBU and DBN.[3] Since it is highly water-soluble, it is easily removed from mixtures in organic solvents. It is also used as a base-catalyst in the production of polyurethane.[4]
gollark: We should make school allow people to choose subjects they are actually interested in.
gollark: Oh, one of the extremely complex quantum thingies, of course.
gollark: What's a TQFT and shut up gnobody.
gollark: Hmm, maybe SPUDNET should do pings every 2500ms instead of 10000ms.
gollark: ...
References
- Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87 (4): 385–395. doi:10.5562/cca2472.
- Rodima, Toomas; Leito, I. (2002). "Acid-Base Equilibria in Nonpolar Media. 2. Self-Consistent Basicity Scale in THF Solution Ranging from 2-Methoxypyridine to EtP1(pyrr) Phosphazene". J. Org. Chem. 67 (6): 1873–1881. doi:10.1021/jo016185p.
- Ishikawa, T.; Kumamoto, T. (2006). "Guanidines in Organic Synthesis". Synthesis. 2006 (5): 737–752. doi:10.1055/s-2006-926325.
- Geoghegan, J. T.; Roth, R. W. (2003). "Catalytic Effects of 1,1,3,3-Tetramethylguanidine for Isocyanate Reactions". J. Appl. Polym. Sci. 9 (3): 1089–1093. doi:10.1002/app.1965.070090325.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.