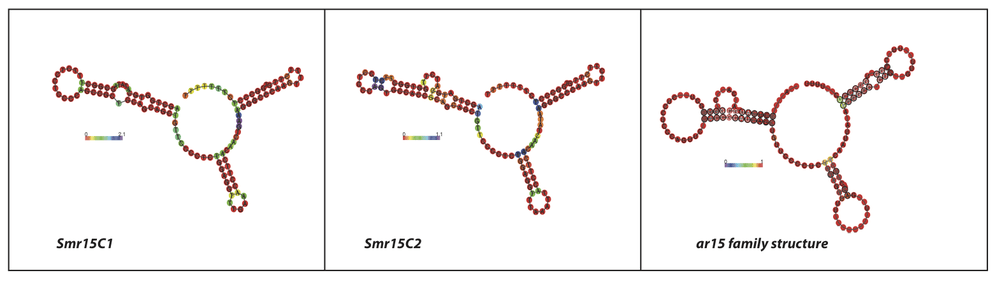
αr15 RNA
αr15 is a family of bacterial small non-coding RNAs with representatives in a broad group of α-proteobacteria from the order Rhizobiales. The first members of this family (smr15C1 and smrC15C2) were found tandemly arranged in the same intergenic region (IGR) of the Sinorhizobium meliloti 1021 chromosome (C).[1] Further homology and structure conservation analysis have identified full-length Smr15C1 and Smr15C2 homologs in several nitrogen-fixing symbiotic rhizobia (i.e. R. leguminosarum bv. viciae, R. leguminosarum bv. trifolii, R. etli, and several Mesorhizobium species), in the plant pathogens belonging to Agrobacterium species (i.e. A. tumefaciens, A. vitis, A. radiobacter, and Agrobacterium H13) as well as in a broad spectrum of Brucella species (B. ovis, B. canis, B. abortus and B. microtis, and several biovars of B. melitensis). The Smr15C1 (115 nt) and Smr15C2 (121 nt) homologs are also encoded in tandem within the same IGR region of Rhizobium and Agrobacterium species, whereas in Brucella species the αr15C loci are spread in the IGRs of Chromosome I. Moreover, this analysis also identified a third αr15 loci in extrachromosomal replicons of the mentioned nitrogen-fixing α-proteobacteria and in the Chromosome II of Brucella species. αr15 RNA species are 99-121 nt long (Table 1) and share a well defined common secondary structure consisting of three stem loops (Figure 1). The transcripts of the αr15 family can be catalogued as trans-acting sRNAs encoded by independent transcription units with recognizable promoter and transcription termination signatures within intergenic regions (IGRs) of the α-proteobacterial genomes (Figure 5).
Discovery and structure
Smr15C1 y Smr15C2 sRNAs were described by del Val et al.,[1] as a result of a computational comparative genomic approach in the intergenic regions (IGRs) of the reference S. meliloti 1021 strain (http://iant.toulouse.inra.fr/bacteria/annotation/cgi/rhime.cgi). Although the primary nucleotide sequence of Smr15C1 y Smr15C2 showed high similarity (84% identity), specific probes for each sRNA could be designed which detected transcripts of different size and expression profiles.[1]
TAP-based 5’-RACE experiments mapped the Smr15C1 and Smr15C2 transcription start sites (TSS) in the S. meliloti 1021 genome (http://iant.toulouse.inra.fr/bacteria/annotation/cgi/rhime.cgi). The Smr15C1 TSS was mapped to the chromosomal position 1698731 nt and the TSS of Smr15C2 to the nt 1698937. The 3’-ends were assumed to be located at the 1698617 nt and 1698817 nt respectively, matching the last residue of the consecutive Us stretch of a bona fide Rho-independent terminator (Figure 5). Parallel and later studies,[2][3] in which Smr15C1 and Smr15C2 transcripts are referred to as two copies of sra41 or Sm3/Sm3', independently confirmed the expression of these sRNAs in S. melilloti and in its closely related strain 2011. Recent deep sequencing-based characterization of the small RNA fraction (50-350 nt) of S. meliloti 2011 also revealed the expression of Smr15C1 and Smr15C2, here referred to as SmelC411 and SmelC412 respectively, mapping the 5’- and 3´-ends of the full-length transcripts to essentially the same positions as del Val et al. in the S. meliloti 1021 chromosome. However, this study identified an additional TSS for Smr15C2 at position 1698948.[4]
The nucleotide sequences of Smr15C1 and Smr15C2 were initially used as query to search against the Rfam database (version 10.0; http://rfam.xfam.org). This search revealed partial homology of both transcripts, restricted to the second hairpin and the Rho-independent terminator, to the RF00519 family of RNAs known as suhB (http://rfam.xfam.org/family/RF00519). However, no structural homologs of the full-length sRNAs were found in this database.
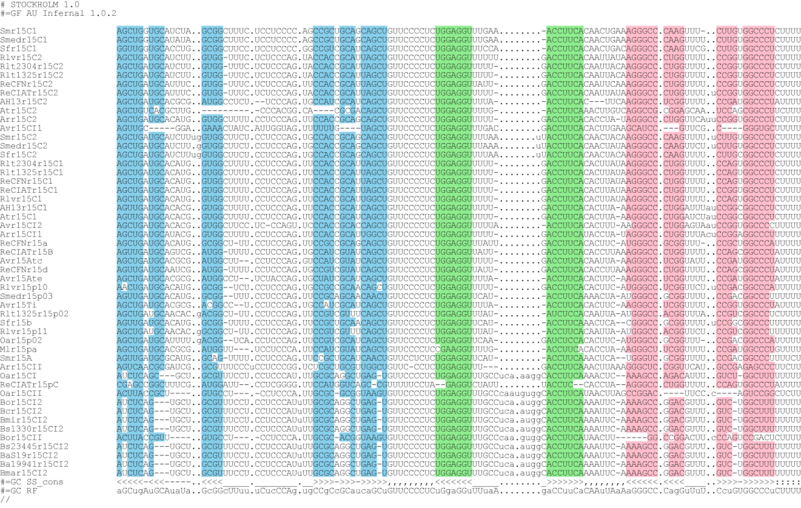
Both S.melilloti αr15 sRNAs were also BLASTed with default parameters against all the currently available bacterial genomes (1,615 sequences at 20 April 2011; https://www.ncbi.nlm.nih.gov). The regions exhibiting significant homology to the query sequence (78-89% similarity) were extracted to create a Covariance Model (CM) from a seed alignment using Infernal (version1.0)[5] (Figure 2). This CM was used in a further search for new members of the αr15 family in the existing bacterial genomic databases.
| CM model | Name | GI accession number | begin | end | strand | %GC | length | Organism |
|---|---|---|---|---|---|---|---|---|
| αr15 | Smr15C1 | gi|15963753|ref|NC_003047.1| | 1698617 | 1698731 | - | 54 | 115 | Sinorhizobium meliloti 1021 |
| αr15 | Smr15C2 | gi|15963753|ref|NC_003047.1| | 1698817 | 1698937 | - | 50 | 121 | Sinorhizobium meliloti 1021 |
| αr15 | Smr15A | gi|16262453|ref|NC_003037.1| | 552873 | 552984 | + | 51 | 112 | Sinorhizobium meliloti 1021 plasmid pSymA |
| αr15 | Smedr15C1 | gi|150395228|ref|NC_009636.1| | 1337011 | 1337126 | - | 53 | 116 | Sinorhizobium medicae WSM419 chromosome |
| αr15 | Smedr15C2 | gi|150395228|ref|NC_009636.1| | 1337212 | 1337331 | - | 50 | 120 | Sinorhizobium medicae WSM419 chromosome |
| αr15 | Smedr15p03 | gi|150378263|ref|NC_009622.1| | 40054 | 40165 | - | 52 | 112 | Sinorhizobium medicae WSM419 plasmid pSMED03 |
| αr15 | Sfr15C1 | gi|227820587|ref|NC_012587.1| | 1612511 | 1612626 | - | 59 | 116 | Sinorhizobium fredii NGR234 chromosome |
| αr15 | Sfr15C2 | gi|227820587|ref|NC_012587.1| | 1612711 | 1612830 | - | 51 | 120 | Sinorhizobium fredii NGR234 chromosome |
| αr15 | Sfr15b | gi|227818258|ref|NC_012586.1| | 134078 | 134190 | - | 55 | 113 | Sinorhizobium fredii NGR234 plasmid pNGR234b |
| αr15 | Atr15C1 | gi|159184118|ref|NC_003062.2| | 2163254 | 2163370 | + | 53 | 117 | Agrobacterium tumefaciens str. C58 chromosome circular |
| αr15 | Atr15C2 | gi|159184118|ref|NC_003062.2| | 2163454 | 2163554 | + | 57 | 101 | Agrobacterium tumefaciens str. C58 chromosome circular |
| αr15 | AH13r15C1 | gi|325291453|ref|NC_015183.1| | 2112823 | 2112939 | + | 52 | 117 | Agrobacterium sp. H13-3 chromosome |
| αr15 | AH13r15C2 | gi|325291453|ref|NC_015183.1| | 2113023 | 2113121 | + | 54 | 99 | Agrobacterium sp. H13-3 chromosome |
| αr15 | AH13r15a | gi|325168279|ref|NC_015184.1| | 211698 | 211807 | - | 53 | 110 | Agrobacterium sp. H13-3 plasmid pAspH13-3a |
| αr15 | ReCIATr15C1 | gi|190889639|ref|NC_010994.1| | 3155217 | 3155332 | + | 51 | 116 | Rhizobium etli CIAT 652 |
| αr15 | ReCIATr15C2 | gi|190889639|ref|NC_010994.1| | 3155440 | 3155555 | + | 48 | 116 | Rhizobium etli CIAT 652 |
| αr15 | ReCIATr15pC | gi|190894340|ref|NC_010997.1| | 941345 | 941452 | + | 53 | 108 | Rhizobium etli CIAT 652 plasmid pC |
| αr15 | ReCIATr15B | gi|190893983|ref|NC_010996.1| | 187927 | 188041 | - | 50 | 115 | Rhizobium etli CIAT 652 plasmid pB |
| αr15 | Arr15CI1 | gi|222084201|ref|NC_011985.1| | 2506215 | 2506331 | + | 52 | 117 | Agrobacterium radiobacter K84 chromosome 1 |
| αr15 | Arr15CI2 | gi|222084201|ref|NC_011985.1| | 2506418 | 2506534 | + | 54 | 117 | Agrobacterium radiobacter K84 chromosome 1 |
| αr15 | Arr15CII | gi|222080781|ref|NC_011983.1| | 1011511 | 1011624 | - | 57 | 114 | Agrobacterium radiobacter K84 chromosome 2 |
| αr15 | Rlt2304r15C1 | gi|209547612|ref|NC_011369.1| | 2770612 | 2770727 | + | 50 | 116 | Rhizobium leguminosarum bv. trifolii WSM2304 chromosome |
| αr15 | Rlt2304r15C2 | gi|209547612|ref|NC_011369.1| | 2770835 | 2770949 | + | 50 | 115 | Rhizobium leguminosarum bv. trifolii WSM2304 chromosome |
| αr15 | Avr15CI1 | gi|222147015|ref|NC_011989.1| | 2608532 | 2608647 | + | 54 | 116 | Agrobacterium vitis S4 chromosome 1 |
| αr15 | Avr15CI2 | gi|222147015|ref|NC_011989.1| | 2608739 | 2608839 | + | 46 | 101 | Agrobacterium vitis S4 chromosome 1 |
| αr15 | Avr15Atc | gi|222083145|ref|NC_011984.1| | 122624 | 122736 | - | 50 | 113 | Agrobacterium vitis S4 plasmid pAtS4c |
| αr15 | Avr15Ate | gi|222102412|ref|NC_011981.1| | 198928 | 199039 | + | 51 | 112 | Agrobacterium vitis S4 plasmid pAtS4e |
| αr15 | Avr15Ti | gi|222080117|ref|NC_011982.1| | 52286 | 52397 | - | 57 | 112 | Agrobacterium vitis S4 plasmid pTiS4 |
| αr15 | Rlvr15C1 | gi|116249766|ref|NC_008380.1| | 3605490 | 3605605 | + | 51 | 116 | Rhizobium leguminosarum bv. viciae 3841 |
| αr15 | Rlvr15C2 | gi|116249766|ref|NC_008380.1| | 3605714 | 3605829 | + | 48 | 116 | Rhizobium leguminosarum bv. viciae 3841 |
| αr15 | Rlvr15p10 | gi|116254467|ref|NC_008381.1| | 138799 | 138912 | + | 56 | 114 | Rhizobium leguminosarum bv. viciae 3841 plasmid pRL10 |
| αr15 | Rlvr15p11 | gi|116255200|ref|NC_008384.1| | 567053 | 567166 | - | 53 | 114 | Rhizobium leguminosarum bv. viciae 3841 plasmid pRL11 |
| αr15 | Rlt1325r15C1 | gi|241202755|ref|NC_012850.1| | 2981275 | 2981390 | + | 50 | 116 | Rhizobium leguminosarum bv. trifolii WSM1325 |
| αr15 | Rlt1325r15C2 | gi|241202755|ref|NC_012850.1| | 2981499 | 2981613 | + | 50 | 115 | Rhizobium leguminosarum bv. trifolii WSM1325 |
| αr15 | Rlt1325r15p02 | gi|241666492|ref|NC_012858.1| | 36176 | 36289 | + | 51 | 114 | Rhizobium leguminosarum bv. trifolii WSM1325 plasmid pR132502 |
| αr15 | ReCFNr15C1 | gi|86355669|ref|NC_007761.1| | 3117667 | 3117782 | + | 50 | 116 | Rhizobium etli CFN 42 |
| αr15 | ReCFNr15C2 | gi|86355669|ref|NC_007761.1| | 3117890 | 3118004 | + | 50 | 115 | Rhizobium etli CFN 42 |
| αr15 | ReCFNr15d | gi|89255298|ref|NC_004041.2| | 172760 | 172874 | - | 50 | 115 | Rhizobium etli CFN 42 symbiotic plasmid p42d |
| αr15 | ReCFNr15a | gi|86359705|ref|NC_007762.1| | 157296 | 157409 | + | 56 | 114 | Rhizobium etli CFN 42 plasmid p42a |
| αr15 | Mlr15a | gi|13488050|ref|NC_002679.1| | 65044 | 65154 | - | 51 | 111 | Mesorhizobium loti MAFF303099 plasmid pMLa |
| αr15 | Bcr15CII | gi|161620094|ref|NC_010104.1| | 707465 | 707572 | + | 56 | 108 | Brucella canis ATCC 23365 chromosome II |
| αr15 | Bcr15CI1 | gi|161617991|ref|NC_010103.1| | 1379297 | 1379398 | - | 51 | 102 | Brucella canis ATCC 23365 chromosome I |
| αr15 | Bcr15CI2 | gi|161617991|ref|NC_010103.1| | 1451969 | 1452087 | - | 50 | 119 | Brucella canis ATCC 23365 chromosome I |
| αr15 | Bs23445r15CI1 | gi|163842277|ref|NC_010169.1| | 1401085 | 1401186 | - | 51 | 102 | Brucella suis ATCC 23445 chromosome I |
| αr15 | Bs23445r15CI2 | gi|163842277|ref|NC_010169.1| | 1473791 | 1473909 | - | 50 | 119 | Brucella suis ATCC 23445 chromosome I |
| αr15 | Bs23445r15CII | gi|163844199|ref|NC_010167.1| | 696081 | 696188 | + | 56 | 108 | Brucella suis ATCC 23445 chromosome II |
| αr15 | Bm16Mr15CI | gi|17986284|ref|NC_003317.1| | 607684 | 607785 | + | 51 | 102 | Brucella melitensis bv. 1 str. 16M chromosome I |
| αr15 | Bm16Mr15CII | gi|17988344|ref|NC_003318.1| | 589501 | 589608 | - | 56 | 108 | Brucella melitensis bv. 1 str. 16M chromosome II |
| αr15 | BaS19r15CII | gi|189022234|ref|NC_010740.1| | 508055 | 508162 | - | 56 | 108 | Brucella abortus S19 chromosome 2 |
| αr15 | BaS19r15CI1 | gi|189023268|ref|NC_010742.1| | 1396794 | 1396895 | - | 51 | 102 | Brucella abortus S19 chromosome 1 |
| αr15 | BaS19r15CI2 | gi|189023268|ref|NC_010742.1| | 1469407 | 1469525 | - | 50 | 119 | Brucella abortus S19 chromosome 1 |
| αr15 | Bm23457r15CII | gi|225685871|ref|NC_012442.1| | 687290 | 687397 | + | 56 | 108 | Brucella melitensis ATCC 23457 chromosome II |
| αr15 | Bm23457r15CI | gi|225851546|ref|NC_012441.1| | 1400641 | 1400742 | - | 51 | 102 | Brucella melitensis ATCC 23457 chromosome I |
| αr15 | Bs1330r15CII | gi|56968493|ref|NC_004311.2| | 708185 | 708292 | + | 56 | 108 | Brucella suis 1330 chromosome II |
| αr15 | Bs1330r15CI1 | gi|56968325|ref|NC_004310.3| | 1380381 | 1380482 | - | 51 | 102 | Brucella suis 1330 chromosome I |
| αr15 | Bs1330r15CI2 | gi|56968325|ref|NC_004310.3| | 1453011 | 1453129 | - | 50 | 119 | Brucella suis 1330 chromosome I |
| αr15 | Ba19941r15CI1 | gi|62288991|ref|NC_006932.1| | 1398464 | 1398565 | - | 51 | 102 | Brucella abortus bv. 1 str. 9-941 chromosome I |
| αr15 | Ba19941r15CI2 | gi|62288991|ref|NC_006932.1| | 1471073 | 1471191 | - | 50 | 119 | Brucella abortus bv. 1 str. 9-941 chromosome I |
| αr15 | Ba19941r15CII | gi|62316961|ref|NC_006933.1| | 508851 | 508958 | - | 56 | 108 | Brucella abortus bv. 1 str. 9-941 chromosome II |
| αr15 | Bmar15CII | gi|83268957|ref|NC_007624.1| | 508839 | 508946 | - | 56 | 108 | Brucella melitensis biovar Abortus 2308 chromosome II |
| αr15 | Bmar15CI1 | gi|82698932|ref|NC_007618.1| | 1395614 | 1395715 | - | 51 | 102 | Brucella melitensis biovar Abortus 2308 chromosome I |
| αr15 | Bmar15CI2 | gi|82698932|ref|NC_007618.1| | 1468227 | 1468345 | - | 50 | 119 | Brucella melitensis biovar Abortus 2308 chromosome I |
| αr15 | Bor15CI1 | gi|148558820|ref|NC_009505.1| | 1387928 | 1388029 | - | 50 | 102 | Brucella ovis ATCC 25840 chromosome I |
| αr15 | Bor15CI2 | gi|148558820|ref|NC_009505.1| | 1460506 | 1460624 | - | 50 | 119 | Brucella ovis ATCC 25840 chromosome I |
| αr15 | Bor15CII | gi|148557829|ref|NC_009504.1| | 709415 | 709524 | + | 54 | 110 | Brucella ovis ATCC 25840 chromosome II |
| αr15 | Bmir15CII | gi|256014795|ref|NC_013118.1| | 709102 | 709209 | + | 56 | 108 | Brucella microti CCM 4915 chromosome 2 |
| αr15 | Bmir15CI1 | gi|256368465|ref|NC_013119.1| | 1387776 | 1387877 | - | 51 | 102 | Brucella microti CCM 4915 chromosome 1 |
| αr15 | Bmir15CI2 | gi|256368465|ref|NC_013119.1| | 1461298 | 1461416 | - | 50 | 119 | Brucella microti CCM 4915 chromosome 1 |
| αr15 | Oar15CI | gi|153007346|ref|NC_009667.1| | 1751482 | 1751598 | + | 49 | 117 | Ochrobactrum anthropi ATCC 49188 chromosome 1 |
| αr15 | Oar15CII | gi|153010078|ref|NC_009668.1| | 1270083 | 1270191 | + | 56 | 109 | Ochrobactrum anthropi ATCC 49188 chromosome 2 |
| αr15 | Oar15p02 | gi|153011934|ref|NC_009670.1| | 22208 | 22320 | + | 54 | 113 | Ochrobactrum anthropi ATCC 49188 plasmid pOANT02 |
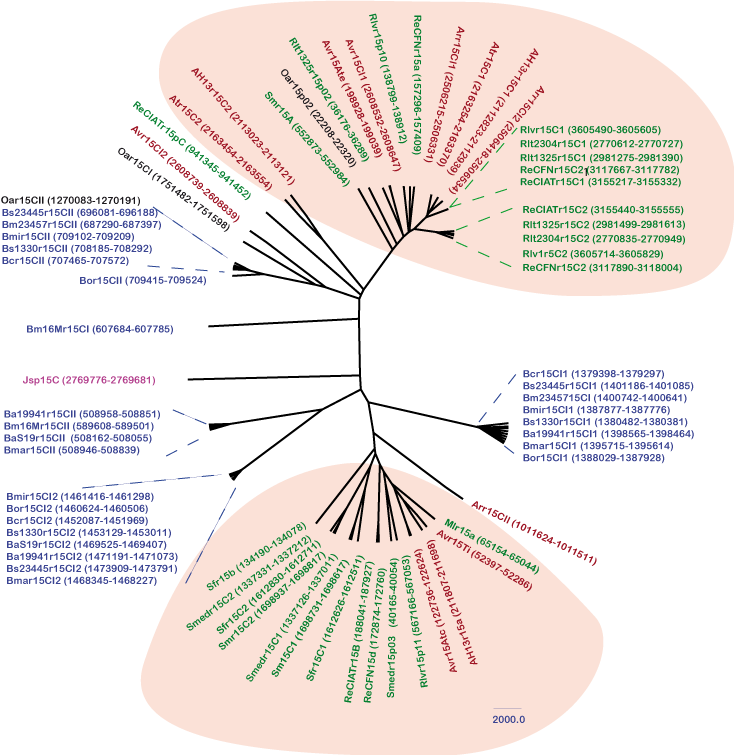
The results were manually inspected to deduce a consensus secondary structure for the family (Figure 1 and Figure 2). The consensus structure was also independently predicted with the program locARNATE[6] comparing the obtained predictions. The manual inspection of the sequences found with the CM using Infernal allowed finding 38 true homologues in phylogenetically related α-proteobacterial genomes. The 26 closest αr15 family members were found as tandem in the same chromosomal IGRs for the following species besides S. melilloti:
- Sinorhizobium species: S. medicae and S. fredii
- Rhizobium species: two R. leguminosarum trifolii strains (WSM304 and WSM35), two R. etli strains CFN 42 and CIAT 652, the reference R. leguminosarum bv. viciae 3841 strain
- Agrobacterium species: A. vitis,A. tumefaciens, A. radiobacter and A. H13
All these sequences showed significant bit scores and Infernal E-values (1.71e-28 - 2.03e-20). However, the plasmidic copies of all mentioned α-proteobacterial genomes and those αr15 members encoded by Brucella species (B. ovis, B. canis, B. abortus, B. microtis, and several biovars of B. melitensis), Ochrobactrum anthropi and Mesorhizobium lotishowed high E-values between (1e-19 and 8e-03) but very low bit-scores.
Expression and functional information
Several studies have assessed Smr15C1 and Smr15C2 expression in S. meliloti 1021 under different biological conditions; i.e. bacterial growth in TY, minimal medium (MM) and luteolin-MM broth and endosymbiotic bacteria (i.e. mature symbiotic alfalfa nodules),[1] high salt stress, oxidative stress and cold and hot shock stresses.[3] The results showed different expression profiles for both sRNAs,[1] which is consistent with their organization in independent and differentially regulated transcription units within the same IGR (Figure 4 and Figure 5).
The expression of Smr15C1 and Smr15C2 in free-living bacteria was found to be growth-dependent but in an opposite manner. While Smr15C1 is accumulated in the stationary phase Smr15C2 is The expression of Smr15C1 and Smr15C2 in free-living bacteria was found to be growth-dependent but in an opposite manner. While Smr15C1 is accumulated in the stationary phase, Smr15C2 is preferentially expressed in log bacterial cultures.[1] Additionally, Schlüter et al.[4] recently described the up-regulation of Smr15C2 under cold shock stress, while no effects of a temperature downshift were observed in the expression of Smr15C1. The growth-dependent opposite expression profiles of Smr15C1 and Smr15C2, have not been observed in their Agrobacterium tumefaciens counterparts referred to as AbcR1 and AbcR2, respectively, by Wilms et al. (Atr15C1 and Atr15C2 in this work). AbcR1 and AbcR2 are induced simultaneously and both accumulate in stationary phase.[9] This behavior agrees with the fact that AbcR1 and AbcR2 have identical promoter-like sequences, being these very similar to the one of Smr15C2, but not to the promoter sequence of Smr15C1 (see Promoter Analysis). Furthermore, a first approach to the function of the AbcR genes revealed that these sRNAs silence the GABA uptake system through the down-regulation of the corresponding ABC transporter genes in an Hfq-dependent manner.[9] GABA is one of the plants signals recognized by rhizobacteria in some plant-bacteria interactions. Thus, these results, point to the shutting off synthesis of the GABA uptake system as a way used by A. tumefaciens to subvert the plant defense mechanism.
Recent co-inmunoprecipitation experiment[10] showed that both, Smr15C1 and Smr15C2, do bind the S. meliloti RNA chaperone Hfq, supporting also a role for these transcripts in this bacterium as trans-acting antisense riboregulators.They were also shown to fine-tune nutrient uptake.[11]
Promoter analysis
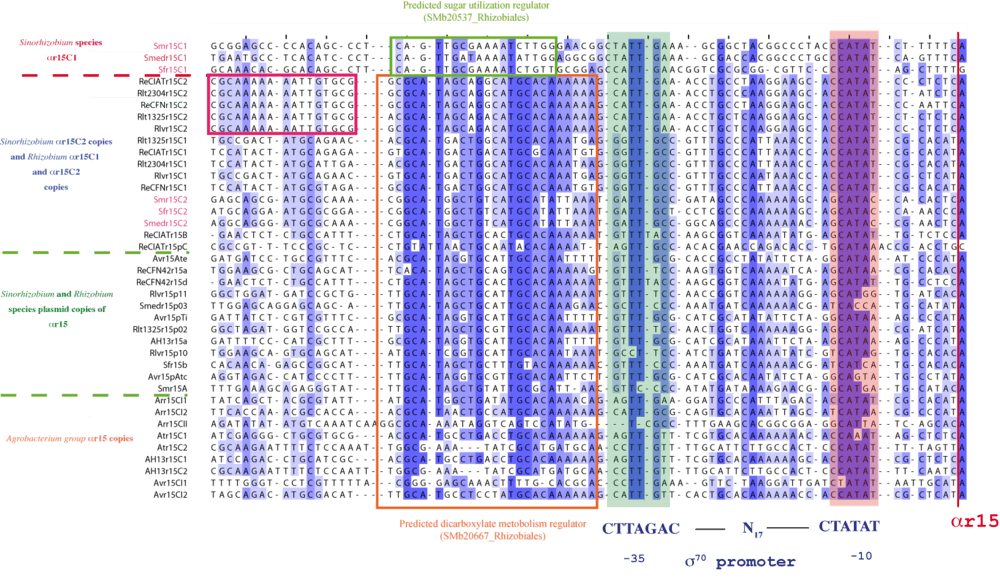
All αr15 loci have recognizable σ70-dependent promoters showing a -35/-10 consensus motif CTTGAC-n17-CTATAT, which has been previously shown to be widely conserved among several other genera in the α-subgroup of proteobacteria.[12] A multiple sequence alignment of these promoter regions revealed a conserved sequence stretch extending up to 80 bp upstream of the transcription start site in all the αr15 loci with the only exceptions of the S. meliloti, S.fredii and S. medicae αr15C1 promoters.
To identify binding sites for other known transcription factors we used the fasta sequences provided by RegPredict[13](http://regpredict.lbl.gov/regpredict/help.html), and used those position weight matrices (PSWM) provided by RegulonDB[14] (http://regulondb.ccg.unam.mx). We built PSWM for each transcription factor from the RegPredict sequences using the Consensus/Patser program, choosing the best final matrix for motif lengths between 14–30 bps a threshold average E-value < 10E-10 for each matrix was established, (see "Thresholded consensus" in http://gps-tools2.its.yale.edu). Moreover, we searched for conserved unknown motifs using MEME[15] (http://meme.sdsc.edu/meme4_6_1/intro.html) and used relaxed regular expressions (i.e. pattern matching) over all αr15 homologs promoters.
These studies revealed a difference in regulation between S. melilloti, S.fredii and S. medicae αr15C1 sRNAs and the rest of the αr15 family members independently of their group of origin (Rhizobium or Agrobacterium ) and genomic location (αr15C1, αr15C2, αr15plasmid) (Figure 4).
The rest of the family members presented a very well conserved 30 bp long region between positions -36 and -75. This conserved region, was used to query databases of known transcription factor motifs with TomTom,[16] the best matching motif was SMb20667_Rhizobiales, motif belonging to RegTransBase (http://regtransbase.lbl.gov/cgi-bin/regtransbase?page=main). The Smb20667 correspond to the binding site for a dicarboxylate metabolism regulator in Rhizobiales that belongs to the LacI family. This motif was identified clustering genes of tartrate dehydrogenase, succinate semialdehyde dehydrogenase, 3-hydroxyisobutyrate dehydrogenase and hydroxypyruvate isomerase in S. meliloti, and several Rhizobiums and it is marked in the promoter alignment figure (Figure 5) with an orange box. Moreover, another conserved sequence was found using MEME in the promoter region of the Sinorhizobium species αr15C1. This conserved region was used to query databases of known transcription factor motifs with TomTom, and the best matching motif was SMb20537_Rhizobiales (Figure 5 in red), that identifies the binding site for a sugar utilization regulator in Rhizobiales from the LacI family as well.
Genomic context
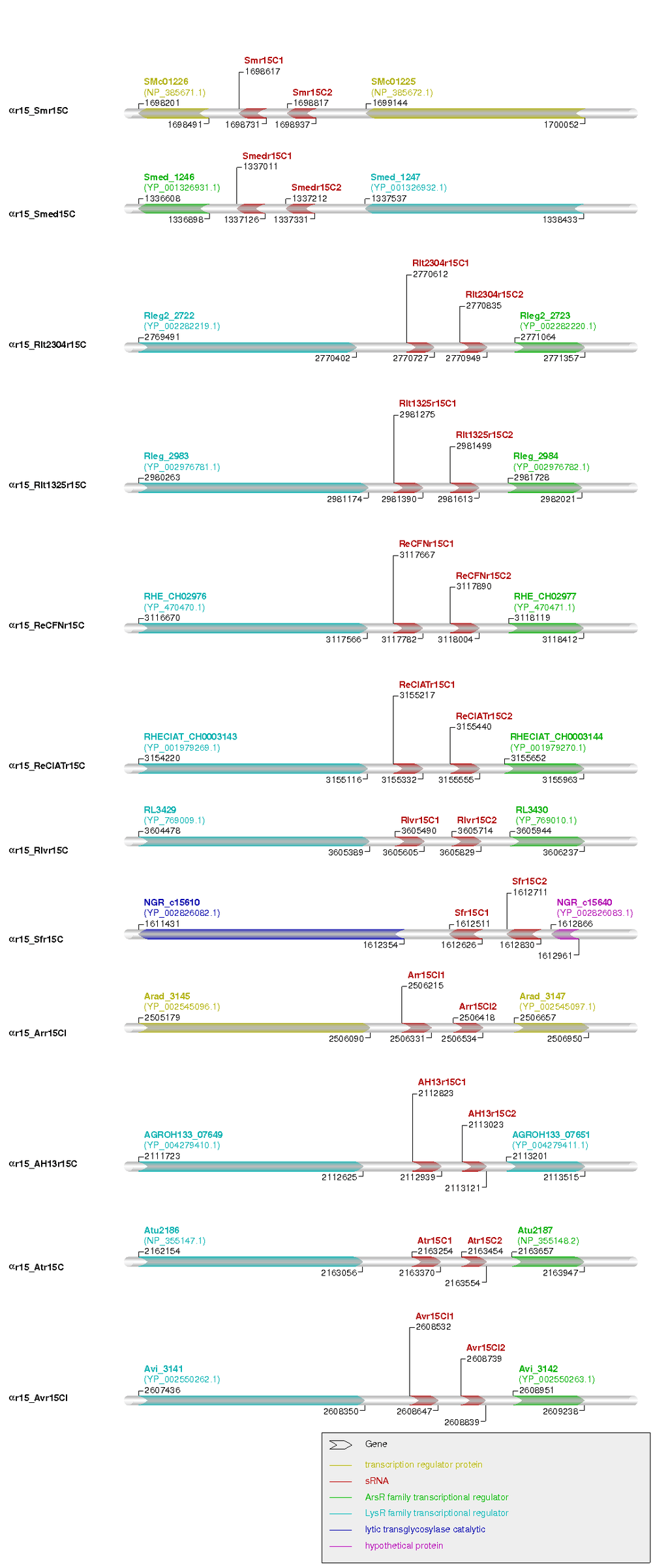
Most of the αr15 family members are trans-encoded sRNAs transcribed from independent promoters in chromosomal IGRs. Many of the αr15 members neighboring genes were not annotated, and thus they were further manually curated.[17][18][19] As a result, we could classify the members of the family in four subgroups according to their genomic context. In the first group are the tandem αr15C1 and αr15C2 loci of the Rhizobium, Sinorhizobium and Agrobacterium species. They exhibited a great degree of conservation in the up and downstream genes, which have been predicted to code for a LysR transcriptional regulator and an AsR transcriptional regulator protein respectively (Figure 5). The only exception in this group was found for S. fredii that presented a very different genomic context. The second group includes the αr15CI1 loci in the Brucella species (additional file 2), which presented a very well conserved genomic context (Aspartate amino transferase and LysR/unknown transcriptional regulator) with partial synteny to the first group. A very different genomic context, not even partially conserved in most cases, was present in all plasmid-borne αr15 loci (additional file 1), which integrates the defined group three, where the flanking genes corresponded to ABC transporter proteins, excisonase or transposase among others. The αr15CI2 loci in the Brucella species (additional file 3) conform the group four and presented an up and downstream conserved genomic context, coding all regions for UDP-3-O-hydroxymyristoyl N-acetylglucosamine deacetylase and GTPAse cell division protein FtsZ. To the last group correspond the αr15CII loci in the Brucella group (additional file 4) where only one of the genes could be annotated always as a glycine deshidrogenase, the other sRNA flanking position was mostly coped by a hypothethical protein conserved in the Brucella group to which no domain, motif or GO functional annotation could be assigned.
Additional Files:
- Genomic context graph of the αr15 family plasmid copies Image:Smbr15 1.png
- Genomic context graph of the αr15CI1 loci in the Brucella group Image:Smbr15 2.png
- Genomic context graph of the αr15CI2 loci in the Brucella group Image:Smbr15 3.png
- Genomic context graph of the αr15CII loci in the Brucella group Image:Smbr15 4.png
| Family | Feature type | Feature name | Strand | Begin | End | Protein name | Annotation | Organism |
|---|---|---|---|---|---|---|---|---|
| αr15_Smr15C | gene | SMc01226 | R | 1698201 | 1698491 | NP_385671.1 | transcription regulator protein | Sinorhizobium meliloti 1021 (NC_003047) |
| αr15_Smr15C | sRNA | Smr15C1 | R | 1698617 | 1698731 | - | sRNA | Sinorhizobium meliloti 1021 (NC_003047) |
| αr15_Smr15C | sRNA | Smr15C2 | R | 1698817 | 1698937 | - | sRNA | Sinorhizobium meliloti 1021 (NC_003047) |
| αr15_Smr15C | gene | SMc01225 | R | 1699144 | 1700052 | NP_385672.1 | transcription regulator protein | Sinorhizobium meliloti 1021 (NC_003047) |
| αr15_Smed15C | gene | Smed_1246 | R | 1336608 | 1336898 | YP_001326931.1 | ArsR family transcriptional regulator | Sinorhizobium medicae WSM419 chromosome (NC_009636) |
| αr15_Smed15C | sRNA | Smedr15C1 | R | 1337011 | 1337126 | - | sRNA | Sinorhizobium medicae WSM419 chromosome (NC_009636) |
| αr15_Smed15C | sRNA | Smedr15C2 | R | 1337212 | 1337331 | - | sRNA | Sinorhizobium medicae WSM419 chromosome (NC_009636) |
| αr15_Smed15C | gene | Smed_1247 | R | 1337537 | 1338433 | YP_001326932.1 | LysR family transcriptional regulator | Sinorhizobium medicae WSM419 chromosome (NC_009636) |
| αr15_Rlt2304r15C | gene | Rleg2_2722 | D | 2769491 | 2770402 | YP_002282219.1 | LysR family transcriptional regulator | Rhizobium leguminosarum bv. trifolii WSM2304 chromosome (NC_011369) |
| αr15_Rlt2304r15C | sRNA | Rlt2304r15C1 | D | 2770612 | 2770727 | - | sRNA | Rhizobium leguminosarum bv. trifolii WSM2304 chromosome (NC_011369) |
| αr15_Rlt2304r15C | sRNA | Rlt2304r15C2 | D | 2770835 | 2770949 | - | sRNA | Rhizobium leguminosarum bv. trifolii WSM2304 chromosome (NC_011369) |
| αr15_Rlt2304r15C | gene | Rleg2_2723 | D | 2771064 | 2771357 | YP_002282220.1 | ArsR family transcriptional regulator | Rhizobium leguminosarum bv. trifolii WSM2304 chromosome (NC_011369) |
| αr15_Rlt1325r15C | gene | Rleg_2983 | D | 2980263 | 2981174 | YP_002976781.1 | LysR family transcriptional regulator | Rhizobium leguminosarum bv. trifolii WSM1325 (NC_012850) |
| αr15_Rlt1325r15C | sRNA | Rlt1325r15C1 | D | 2981275 | 2981390 | - | sRNA | Rhizobium leguminosarum bv. trifolii WSM1325 (NC_012850) |
| αr15_Rlt1325r15C | sRNA | Rlt1325r15C2 | D | 2981499 | 2981613 | - | sRNA | Rhizobium leguminosarum bv. trifolii WSM1325 (NC_012850) |
| αr15_Rlt1325r15C | gene | Rleg_2984 | D | 2981728 | 2982021 | YP_002976782.1 | ArsR family transcriptional regulator | Rhizobium leguminosarum bv. trifolii WSM1325 (NC_012850) |
| αr15_ReCFNr15C | gene | RHE_CH02976 | D | 3116670 | 3117566 | YP_470470.1 | LysR family transcriptional regulator | Rhizobium etli CFN 42 (NC_007761) |
| αr15_ReCFNr15C | sRNA | ReCFNr15C1 | D | 3117667 | 3117782 | - | sRNA | Rhizobium etli CFN 42 (NC_007761) |
| αr15_ReCFNr15C | sRNA | ReCFNr15C2 | D | 3117890 | 3118004 | - | sRNA | Rhizobium etli CFN 42 (NC_007761) |
| αr15_ReCFNr15C | gene | RHE_CH02977 | D | 3118119 | 3118412 | YP_470471.1 | ArsR family transcriptional regulator | Rhizobium etli CFN 42 (NC_007761) |
| αr15_ReCIATr15C | gene | RHECIAT_CH0003143 | D | 3154220 | 3155116 | YP_001979269.1 | LysR family transcriptional regulator | Rhizobium etli CIAT 652 (NC_010994) |
| αr15_ReCIATr15C | sRNA | ReCIATr15C1 | D | 3155217 | 3155332 | - | sRNA | Rhizobium etli CIAT 652 (NC_010994) |
| αr15_ReCIATr15C | sRNA | ReCIATr15C2 | D | 3155440 | 3155555 | - | sRNA | Rhizobium etli CIAT 652 (NC_010994) |
| αr15_ReCIATr15C | gene | RHECIAT_CH0003144 | D | 3155652 | 3155963 | YP_001979270.1 | ArsR family transcriptional regulator | Rhizobium etli CIAT 652 (NC_010994) |
| αr15_Rlvr15C | gene | RL3429 | D | 3604478 | 3605389 | YP_769009.1 | LysR family transcriptional regulator | Rhizobium leguminosarum bv. viciae 3841 (NC_008380) |
| αr15_Rlvr15C | sRNA | Rlvr15C1 | D | 3605490 | 3605605 | - | sRNA | Rhizobium leguminosarum bv. viciae 3841 (NC_008380) |
| αr15_Rlvr15C | sRNA | Rlvr15C2 | D | 3605714 | 3605829 | - | sRNA | Rhizobium leguminosarum bv. viciae 3841 (NC_008380) |
| αr15_Rlvr15C | gene | RL3430 | D | 3605944 | 3606237 | YP_769010.1 | ArsR family transcriptional regulator | Rhizobium leguminosarum bv. viciae 3841 (NC_008380) |
| αr15_Sfr15C | gene | NGR_c15610 | R | 1611431 | 1612354 | YP_002826082.1 | lytic transglycosylase catalytic | Sinorhizobium fredii NGR234 chromosome (NC_012587) |
| αr15_Sfr15C | sRNA | Sfr15C1 | R | 1612511 | 1612626 | - | sRNA | Sinorhizobium fredii NGR234 chromosome (NC_012587) |
| αr15_Sfr15C | sRNA | Sfr15C2 | R | 1612711 | 1612830 | - | sRNA | Sinorhizobium fredii NGR234 chromosome (NC_012587) |
| αr15_Sfr15C | gene | NGR_c15640 | R | 1612866 | 1612961 | YP_002826083.1 | hypothetical protein | Sinorhizobium fredii NGR234 chromosome (NC_012587) |
| αr15_Arr15CI | gene | Arad_3145 | D | 2505179 | 2506090 | YP_002545096.1 | transcription regulator protein | Agrobacterium radiobacter K84 chromosome 1 (NC_011985) |
| αr15_Arr15CI | sRNA | Arr15CI1 | D | 2506215 | 2506331 | - | sRNA | Agrobacterium radiobacter K84 chromosome 1 (NC_011985) |
| αr15_Arr15CI | sRNA | Arr15CI2 | D | 2506418 | 2506534 | - | sRNA | Agrobacterium radiobacter K84 chromosome 1 (NC_011985) |
| αr15_Arr15CI | gene | Arad_3147 | D | 2506657 | 2506950 | YP_002545097.1 | transcription regulator protein | Agrobacterium radiobacter K84 chromosome 1 (NC_011985) |
| αr15_AH13r15C | gene | AGROH133_07649 | D | 2111723 | 2112625 | YP_004279410.1 | LysR family transcriptional regulator | Agrobacterium sp. H13-3 chromosome (NC_015183) |
| αr15_AH13r15C | sRNA | AH13r15C1 | D | 2112823 | 2112939 | - | sRNA | Agrobacterium sp. H13-3 chromosome (NC_015183) |
| αr15_AH13r15C | sRNA | AH13r15C2 | D | 2113023 | 2113121 | - | sRNA | Agrobacterium sp. H13-3 chromosome (NC_015183) |
| αr15_AH13r15C | gene | AGROH133_07651 | D | 2113201 | 2113515 | YP_004279411.1 | LysR family transcriptional regulator | Agrobacterium sp. H13-3 chromosome (NC_015183) |
| αr15_Atr15C | gene | Atu2186 | D | 2162154 | 2163056 | NP_355147.1 | LysR family transcriptional regulator | Agrobacterium tumefaciens C58 chromosome circular (NC_003062) |
| αr15_Atr15C | sRNA | Atr15C1 | D | 2163254 | 2163370 | - | sRNA | Agrobacterium tumefaciens C58 chromosome circular (NC_003062) |
| αr15_Atr15C | sRNA | Atr15C2 | D | 2163454 | 2163554 | - | sRNA | Agrobacterium tumefaciens C58 chromosome circular (NC_003062) |
| αr15_Atr15C | gene | Atu2187 | D | 2163657 | 2163947 | NP_355148.2 | ArsR family transcriptional regulator | Agrobacterium tumefaciens C58 chromosome circular (NC_003062) |
| αr15_Avr15CI | gene | Avi_3141 | D | 2607436 | 2608350 | YP_002550262.1 | LysR family transcriptional regulator | Agrobacterium vitis S4 chromosome 1 (NC_011989) |
| αr15_Avr15CI | sRNA | Avr15CI1 | D | 2608532 | 2608647 | - | sRNA | Agrobacterium vitis S4 chromosome 1 (NC_011989) |
| αr15_Avr15CI | sRNA | Avr15CI2 | D | 2608739 | 2608839 | - | sRNA | Agrobacterium vitis S4 chromosome 1 (NC_011989) |
| αr15_Avr15CI | gene | Avi_3142 | D | 2608951 | 2609238 | YP_002550263.1 | ArsR family transcriptional regulator | Agrobacterium vitis S4 chromosome 1 (NC_011989) |
| αr15_ReCFNr15a | gene | RHE_PA00141 | D | 152078 | 157177 | YP_471730.1 | n-6 DNA methylase | Rhizobium etli CFN 42 plasmid p42a (NC_007762) |
| αr15_ReCFNr15a | sRNA | ReCFNr15a | D | 157296 | 157409 | - | sRNA | Rhizobium etli CFN 42 plasmid p42a (NC_007762) |
| αr15_ReCFNr15a | gene | RHE_PA00143 | D | 157744 | 159486 | YP_471732.1 | plasmid partitioning protein | Rhizobium etli CFN 42 plasmid p42a (NC_007762) |
| αr15_ReCIATr15B | gene | RHECIAT_PB0000171 | R | 187267 | 187788 | YP_001984434.1 | excisonase protein | Rhizobium etli CIAT 652 plasmid pB (NC_010996) |
| αr15_ReCIATr15B | sRNA | ReCIATr15B | R | 187927 | 188041 | - | sRNA | Rhizobium etli CIAT 652 plasmid pB (NC_010996) |
| αr15_ReCIATr15B | gene | RHECIAT_PB0000172 | R | 188226 | 189416 | YP_001984435.1 | hypothetical protein | Rhizobium etli CIAT 652 plasmid pB (NC_010996) |
| αr15_Avr15Atc | gene | Avi_9155 | D | 121509 | 122600 | YP_002542647.1 | DNA polymerase III alpha chain | Agrobacterium vitis S4 plasmid pAtS4c (NC_011984) |
| αr15_Avr15Atc | sRNA | Avr15Atc | R | 122624 | 122736 | - | sRNA | Agrobacterium vitis S4 plasmid pAtS4c (NC_011984) |
| αr15_Avr15Atc | gene | Avi_9156 | D | 123011 | 123358 | YP_002542648.1 | helicase subunit of the DNA excision repair complex | Agrobacterium vitis S4 plasmid pAtS4c (NC_011984) |
| αr15_ReCFNr15d | gene | RHE_PD00155 | R | 172105 | 172731 | NP_659882.1 | excisonase protein | Rhizobium etli CFN 42 symbiotic plasmid p42d (NC_004041) |
| αr15_ReCFNr15d | sRNA | ReCFNr15d | R | 172760 | 172874 | - | sRNA | Rhizobium etli CFN 42 symbiotic plasmid p42d (NC_004041) |
| αr15_ReCFNr15d | gene | RHE_PD00156 | R | 173058 | 174248 | NP_659881.2 | hypothetical protein | Rhizobium etli CFN 42 symbiotic plasmid p42d (NC_004041) |
| αr15_Avr15Ate | gene | Avi_7235 | D | 198046 | 198699 | YP_002539630.1 | ABC transporter membrane spanning protein | Agrobacterium vitis S4 plasmid pAtS4e (NC_011981) |
| αr15_Avr15Ate | sRNA | Avr15Ate | D | 198928 | 199039 | - | sRNA | Agrobacterium vitis S4 plasmid pAtS4e (NC_011981) |
| αr15_Avr15Ate | gene | Avi_7237 | D | 199739 | 200020 | YP_002539631.1 | transposase | Agrobacterium vitis S4 plasmid pAtS4e (NC_011981) |
| αr15_AH13r15a | gene | AGROH133_14527 | R | 210807 | 211133 | YP_004280311.1 | XRE family transcriptional regulator | Agrobacterium sp. H13-3 plasmid pAspH13-3a (NC_015184) |
| αr15_AH13r15a | gene | AGROH133_14529 | R | 211195 | 211713 | - | addiction module toxin | Agrobacterium sp. H13-3 plasmid pAspH13-3a (NC_015184) |
| αr15_AH13r15a | sRNA | AH13r15a | R | 211698 | 211807 | - | sRNA | Agrobacterium sp. H13-3 plasmid pAspH13-3a (NC_015184) |
| αr15_AH13r15a | gene | AGROH133_14530 | R | 211828 | 212286 | YP_004280313.1 | hypothetical protein | Agrobacterium sp. H13-3 plasmid pAspH13-3a (NC_015184) |
| αr15_Smedr15p03 | gene | Smed_6375 | R | 39464 | 39784 | YP_001314894.1 | XRE family transcriptional regulator | Sinorhizobium medicae WSM419 plasmid pSMED03 (NC_009622) |
| αr15_Smedr15p03 | sRNA | Smedr15p03 | R | 40054 | 40165 | - | sRNA | Sinorhizobium medicae WSM419 plasmid pSMED03 (NC_009622) |
| αr15_Smedr15p03 | gene | Smed_6376 | D | 40123 | 40425 | - | hypothetical protein | Sinorhizobium medicae WSM419 plasmid pSMED03 (NC_009622) |
| αr15_Avr15Ti | gene | Avi_8074 | R | 51395 | 51682 | YP_002540018.1 | XRE family transcriptional regulator | Agrobacterium vitis S4 plasmid pTiS4 (NC_011982) |
| αr15_Avr15Ti | sRNA | Avr15Ti | R | 52286 | 52397 | - | sRNA | Agrobacterium vitis S4 plasmid pTiS4 (NC_011982) |
| αr15_Avr15Ti | gene | Avi_8076 | D | 52672 | 53013 | YP_002540020.1 | helicase subunit of the DNA excision repair complex | Agrobacterium vitis S4 plasmid pTiS4 (NC_011982) |
| αr15_Rlt1325r15p02 | gene | Rleg_6607 | D | 35260 | 35928 | YP_002984610.1 | hypothetical protein | Rhizobium leguminosarum bv. trifolii WSM1325 plasmid pRt132502 (NC_012858) |
| αr15_Rlt1325r15p02 | sRNA | Rlt1325r15p02 | D | 36176 | 36289 | - | sRNA | Rhizobium leguminosarum bv. trifolii WSM1325 plasmid pRt132502 (NC_012858) |
| αr15_Rlt1325r15p02 | gene | Rleg_6608 | D | 36740 | 37528 | YP_002984611.1 | Exonuclease RNase T and DNA polymerase II | Rhizobium leguminosarum bv. trifolii WSM1325 plasmid pRt132502 (NC_012858) |
| αr15_Sfr15b | gene | NGR_b01430 | D | 133718 | 133900 | YP_002822362.1 | hypothetical protein | Sinorhizobium fredii NGR234 plasmid pNGR234b (NC_012586) |
| αr15_Sfr15b | sRNA | Sfr15b | R | 134078 | 134190 | - | sRNA | Sinorhizobium fredii NGR234 plasmid pNGR234b (NC_012586) |
| αr15_Sfr15b | gene | NGR_b01450 | R | 134349 | 136811 | YP_002822364.1 | diguanylate cyclase phosphodiesterase with pas pac sensor | Sinorhizobium fredii NGR234 plasmid pNGR234b (NC_012586) |
| αr15_Rlvr15p11 | gene | pRL110525 | R | 566752 | 566955 | YP_771559.1 | hypothetical protein | Rhizobium leguminosarum bv. viciae 3841 plasmid pRL11 (NC_008384) |
| αr15_Rlvr15p11 | sRNA | Rlvr15p11 | R | 567053 | 567166 | - | sRNA | Rhizobium leguminosarum bv. viciae 3841 plasmid pRL11 (NC_008384) |
| αr15_Rlvr15p11 | gene | pRL110526 | R | 567397 | 568065 | YP_771560.1 | hypothetical protein | Rhizobium leguminosarum bv. viciae 3841 plasmid pRL11 (NC_008384) |
| αr15_Oar15p02 | gene | Oant_4749 | D | 21546 | 21971 | YP_001373166.1 | PilT domain-containing protein | Ochrobactrum anthropi ATCC 49188 plasmid pOANT02 (NC_009670) |
| αr15_Oar15p02 | sRNA | Oar15p02 | D | 22208 | 22320 | - | sRNA | Ochrobactrum anthropi ATCC 49188 plasmid pOANT02 (NC_009670) |
| αr15_Oar15p02 | gene | Oant_4750 | D | 22661 | 24454 | YP_001373167.1 | plasmid partitioning protein | Ochrobactrum anthropi ATCC 49188 plasmid pOANT02 (NC_009670) |
| αr15_Mlr15a | gene | msl9071 | R | 64650 | 64817 | NP_085644.1 | hypothetical protein | Mesorhizobium loti MAFF303099 plasmid pMLa (NC_002679) |
| αr15_Mlr15a | sRNA | Mlr15a | R | 65044 | 65154 | - | sRNA | Mesorhizobium loti MAFF303099 plasmid pMLa (NC_002679) |
| αr15_Mlr15a | gene | msl9074 | R | 65712 | 66008 | NP_085645.1 | hypothetical protein | Mesorhizobium loti MAFF303099 plasmid pMLa (NC_002679) |
| αr15_Smr15A | gene | SMa0995 | R | 551249 | 552481 | NP_435782.1 | transposase | Sinorhizobium meliloti 1021 plasmid pSymA (NC_003037) |
| αr15_Smr15A | sRNA | Smr15A | D | 552873 | 552984 | - | sRNA | Sinorhizobium meliloti 1021 plasmid pSymA (NC_003037) |
| αr15_Smr15A | gene | SMa0997 | D | 553196 | 553492 | NP_435783.1 | transposase | Sinorhizobium meliloti 1021 plasmid pSymA (NC_003037) |
| αr15_Arr15CII | gene | Arad_8155 | D | 1010459 | 1011472 | YP_002541089.1 | hypothetical protein | Agrobacterium radiobacter K84 chromosome 2 (NC_011983) |
| αr15_Arr15CII | sRNA | Arr15CII | R | 1011511 | 1011624 | - | sRNA | Agrobacterium radiobacter K84 hromosome 2 (NC_011983) |
| αr15_Arr15CII | gene | Arad_8157 | D | 1012367 | 1013791 | YP_002541091.1 | monooxygenase protein | Agrobacterium radiobacter K84 hromosome 2 (NC_011983) |
| αr15_Oar15CI | gene | Oant_1670 | R | 1750252 | 1751097 | YP_001370215.1 | metallophosphoesterase | Ochrobactrum anthropi ATCC 49188 chromosome 1 (NC_009667) |
| αr15_Oar15CI | sRNA | Oar15CI | D | 1751482 | 1751598 | - | sRNA | Ochrobactrum anthropi ATCC 49188 chromosome 1 (NC_009667) |
| αr15_Oar15CI | gene | Oant_1671 | D | 1752063 | 1753466 | YP_001370216.1 | RNA-directed DNA polymerase | Ochrobactrum anthropi ATCC 49188 chromosome 1 (NC_009667) |
| αr15_ReCIATr15pC | gene | RHECIAT_PC0000855 | R | 938497 | 939639 | YP_001985475.1 | acyltransferase 3 | Rhizobium etli CIAT 652 plasmid pC (NC_010997) |
| αr15_ReCIATr15pC | sRNA | ReCIATr15pC | D | 941345 | 941452 | - | sRNA | Rhizobium etli CIAT 652 plasmid pC (NC_010997) |
| αr15_ReCIATr15pC | gene | RHECIAT_PC0000856 | R | 941811 | 945572 | YP_001985476.1 | hemolysin-type calcium-binding protein | Rhizobium etli CIAT 652 plasmid pC (NC_010997) |
| αr15_Oar15CII | gene | Oant_3861 | R | 1269354 | 1269818 | YP_001372395.1 | hypothetical protein | Ochrobactrum anthropi ATCC 49188 chromosome 2 (NC_009668) |
| αr15_Oar15CII | sRNA | Oar15CII | D | 1270083 | 1270191 | - | sRNA | Ochrobactrum anthropi ATCC 49188 chromosome 2 (NC_009668) |
| αr15_Oar15CII | gene | Oant_3862 | R | 1270409 | 1273222 | YP_001372396.1 | glycine dehydrogenase | Ochrobactrum anthropi ATCC 49188 chromosome 2 (NC_009668) |
| αr15_Bm23445r15CII | gene | BSUIS_B0713 | R | 695738 | 695851 | YP_001622510.1 | hypothetical protein | Brucella suis ATCC 23445 chromosome II (NC_010167) |
| αr15_Bm23445r15CII | sRNA | Bm23445r15CII | D | 696081 | 696188 | - | sRNA | Brucella suis ATCC 23445 chromosome II (NC_010167) |
| αr15_Bm23445r15CII | gene | BSUIS_B0714 | D | 696129 | 696196 | - | unknown | Brucella suis ATCC 23445 chromosome II (NC_010167) |
| αr15_Bm23445r15CII | gene | BSUIS_B0715 | R | 696787 | 699585 | YP_001622511.1 | glycine dehydrogenase | Brucella suis ATCC 23445 chromosome II (NC_010167) |
| αr15_Bm16Mr15CII | gene | BMEII0561 | D | 586104 | 588902 | NP_541539.1 | glycine dehydrogenase | Brucella melitensis bv. 1 str. 16M chromosome II (NC_003318) |
| αr15_Bm16Mr15CII | sRNA | Bm16Mr15CII | R | 589501 | 589608 | - | sRNA | Brucella melitensis bv. 1 str. 16M chromosome II (NC_003318) |
| αr15_Bm16Mr15CII | gene | BMEII0562 | D | 589690 | 589950 | NP_541540.1 | hypothetical protein | Brucella melitensis bv. 1 str. 16M chromosome II (NC_003318) |
| αr15_BaS19r15CII | gene | BAbS19_II04850 | D | 504658 | 507456 | YP_001932428.1 | glycine dehydrogenase | Brucella abortus S19 chromosome 2 (NC_010740) |
| αr15_BaS19r15CII | sRNA | BaS19r15CII | R | 508055 | 508162 | - | sRNA | Brucella abortus S19 chromosome 2 (NC_010740) |
| αr15_BaS19r15CII | gene | BAbS19_II04860 | D | 508392 | 508505 | YP_001932429.1 | hypothetical protein | Brucella abortus S19 chromosome 2 (NC_010740) |
| αr15_Bm23457r15CII | gene | BMEA_B0698 | D | 686472 | 686966 | YP_002734467.1 | proline dehydrogenase transcriptional activator | Brucella melitensis ATCC 23457 chromosome II (NC_012442) |
| αr15_Bm23457r15CII | sRNA | Bm23457r15CII | D | 687290 | 687397 | - | sRNA | Brucella melitensis ATCC 23457 chromosome II (NC_012442) |
| αr15_Bm23457r15CII | gene | BMEA_B0701 | R | 687996 | 690794 | YP_002734468.1 | glycine dehydrogenase | Brucella melitensis ATCC 23457 chromosome II (NC_012442) |
| αr15_Bmir15CII | gene | BMI_II717 | R | 708784 | 709020 | YP_003105497.1 | hypothetical protein | Brucella microti CCM 4915 chromosome 2 (NC_013118) |
| αr15_Bmir15CII | sRNA | Bmir15CII | D | 709102 | 709209 | - | sRNA | Brucella microti CCM 4915 chromosome 2 (NC_013118) |
| αr15_Bmir15CII | gene | BMI_II718 | R | 709832 | 712630 | YP_003105498.1 | glycine dehydrogenase | Brucella microti CCM 4915 chromosome 2 (NC_013118) |
| αr15_Bs1330r15CII | gene | BRA0724 | R | 707842 | 707955 | NP_699901.1 | hypothetical protein | Brucella suis 1330 chromosome II (NC_004311) |
| αr15_Bs1330r15CII | sRNA | Bs1330r15CII | D | 708185 | 708292 | - | sRNA | Brucella suis 1330 chromosome II (NC_004311) |
| αr15_Bs1330r15CII | gene | BRA0725 | R | 708891 | 711689 | NP_699902.1 | glycine dehydrogenase | Brucella suis 1330 chromosome II (NC_004311) |
| αr15_Ba19941r15CII | gene | BruAb2_0506 | D | 505454 | 508252 | YP_223286.1 | glycine dehydrogenase | Brucella abortus bv. 1 str. 9-941 chromosome II (NC_006933) |
| αr15_Ba19941r15CII | sRNA | Ba19941r15CII | R | 508851 | 508958 | - | sRNA | Brucella abortus bv. 1 str. 9-941 chromosome II (NC_006933) |
| αr15_Ba19941r15CII | gene | BruAb2_0507 | D | 509188 | 509301 | YP_223287.1 | hypothetical protein | Brucella abortus bv. 1 str. 9-941 chromosome II (NC_006933) |
| αr15_Bmαr15CII | gene | BAB2_0515 | D | 505442 | 508240 | YP_418705.1 | glycine dehydrogenase | Brucella melitensis biovar Abortus 2308 chromosome II (NC_007624) |
| αr15_Bmαr15CII | sRNA | Bmαr15CII | R | 508839 | 508946 | - | sRNA | Brucella melitensis biovar Abortus 2308 chromosome II (NC_007624) |
| αr15_Bmαr15CII | gene | BAB2_0516 | D | 509028 | 509264 | YP_418706.1 | hypothetical protein | Brucella melitensis biovar Abortus 2308 chromosome II (NC_007624) |
| αr15_Bcr15CII | gene | BCAN_B0729 | D | 706646 | 707140 | YP_001594668.1 | proline dehydrogenase transcriptional activator | Brucella canis ATCC 23365 chromosome II (NC_010104) |
| αr15_Bcr15CII | sRNA | Bcr15CII | D | 707465 | 707572 | - | sRNA | Brucella canis ATCC 23365 chromosome II (NC_010104) |
| αr15_Bcr15CII | gene | BCAN_B0730 | R | 708171 | 710969 | YP_001594669.1 | glycine dehydrogenase | Brucella canis ATCC 23365 chromosome II (NC_010104) |
| αr15_Bor15CI2 | gene | BOV_1448 | D | 1459218 | 1460420 | YP_001259376.1 | aspartate aminotransferase | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
| αr15_Bor15CI2 | sRNA | Bor15CI2 | R | 1460506 | 1460624 | - | sRNA | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
| αr15_Bor15CI2 | gene | BOV_1449 | R | 1460890 | 1461795 | YP_001259377.1 | LysR family transcriptional regulator | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
| αr15_Bcr15CI2 | gene | BCAN_A1532 | D | 1450681 | 1451883 | YP_001593329.1 | aspartate aminotransferase | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bcr15CI2 | sRNA | Bcr15CI2 | R | 1451969 | 1452087 | - | sRNA | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bcr15CI2 | gene | BCAN_A1535 | R | 1452353 | 1453258 | YP_001593332.1 | transcription regulator protein | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bmir15CI2 | gene | BMI_I1510 | D | 1460010 | 1461212 | YP_003107423.1 | aspartate aminotransferase | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bmir15CI2 | sRNA | Bmir15CI2 | R | 1461298 | 1461416 | - | sRNA | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bmir15CI2 | gene | BMI_I1512 | R | 1461682 | 1462587 | YP_003107425.1 | LysR family transcriptional regulator | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bs1330r15CI2 | gene | BR1495 | D | 1451723 | 1452925 | NP_698491.1 | aspartate aminotransferase | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Bs1330r15CI2 | sRNA | Bs1330r15CI2 | R | 1453011 | 1453129 | - | sRNA | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Bs1330r15CI2 | gene | BR1498 | R | 1453395 | 1454300 | NP_698494.1 | LysR family transcriptional regulator | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Bor15CII | sRNA | Bor15CII | D | 709415 | 709524 | - | sRNA | Brucella ovis ATCC 25840 chromosome II (NC_009504) |
| αr15_Bor15CII | gene | BOV_A0677 | R | 704750 | 708432 | - | proline dehydrogenase transcriptional activator | Brucella ovis ATCC 25840 chromosome II (NC_009504) |
| αr15_Bor15CII | gene | BOV_A0679 | R | 710122 | 712920 | YP_001257680.1 | glycine dehydrogenas | Brucella ovis ATCC 25840 chromosome II (NC_009504) |
| αr15_Bm23445r15CI2 | gene | BSUIS_A1552 | D | 1472504 | 1473706 | YP_001628154.1 | aspartate aminotransferase | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_Bm23445r15CI2 | sRNA | Bm23445r15CI2 | R | 1473791 | 1473909 | - | sRNA | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_Bm23445r15CI2 | gene | BSUIS_A1554 | R | 1474175 | 1475080 | YP_001628156.1 | hypothetical protein | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_BaS19r15CI2 | gene | BAbS19_I14120 | D | 1468120 | 1469322 | YP_001935379.1 | aspartate aminotransferase | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_BaS19r15CI2 | sRNA | BaS19r15CI2 | R | 1469407 | 1469525 | - | sRNA | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_BaS19r15CI2 | gene | BAbS19_I14130 | D | 1469632 | 1469739 | YP_001935380.1 | hypothetical protein | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_Ba19941r15CI2 | gene | BruAb1_1488 | D | 1469786 | 1470988 | YP_222177.1 | aspartate aminotransferase | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Ba19941r15CI2 | sRNA | Ba19941r15CI2 | R | 1471073 | 1471191 | - | sRNA | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Ba19941r15CI2 | gene | BruAb1_1490 | D | 1471190 | 1471405 | YP_222179.1 | hypothetical protein | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Bmαr15CI2 | gene | BAB1_1514 | D | 1466940 | 1468142 | YP_414880.1 | aspartate aminotransferase | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Bmαr15CI2 | sRNA | Bmαr15CI2 | R | 1468227 | 1468345 | - | sRNA | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Bmαr15CI2 | gene | BAB1_1516 | D | 1468344 | 1468559 | YP_414882.1 | hypothetical protein | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Bcr15CI1 | gene | BCAN_A1457 | R | 1377955 | 1378815 | YP_001593259.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bcr15CI1 | sRNA | Bcr15CI1 | R | 1379297 | 1379398 | - | sRNA | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bcr15CI1 | sRNA | BCAN_A1458 | R | 1379355 | 1381055 | YP_001593260.1 | cell division protein Fts | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bcr15CI1 | gene | BCAN_A1459 | R | 1381152 | 1382474 | YP_001593261.1 | cell division protein Fts | Brucella canis ATCC 23365 chromosome I (NC_010103) |
| αr15_Bm23445r15CI1 | gene | BSUIS_A1476 | D | 1400706 | 1400831 | YP_001628084.1 | hypothetical protein | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_Bm23445r15CI1 | sRNA | Bm23445r15CI1 | R | 1401085 | 1401186 | - | sRNA | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_Bm23445r15CI1 | gene | BSUIS_A1477 | R | 1401143 | 1402843 | YP_001628085.1 | cell division protein Fts | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_Bm23445r15CI1 | gene | BSUIS_A1478 | R | 1402940 | 1404262 | YP_001628086.1 | cell division protein Fts | Brucella suis ATCC 23445 chromosome I (NC_010169) |
| αr15_Bm16Mr15CI | gene | BMEI0585 | D | 606025 | 607641 | NP_539502.1 | cell division protein Fts | Brucella melitensis bv. 1 str. 16M chromosome I (NC_003317) |
| αr15_Bm16Mr15CI | sRNA | Bm16Mr15CI | D | 607684 | 607785 | - | sRNA | Brucella melitensis bv. 1 str. 16M chromosome I (NC_003317) |
| αr15_Bm16Mr15CI | gene | BMEI0586 | D | 608267 | 609127 | NP_539503.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella melitensis bv. 1 str. 16M chromosome I (NC_003317) |
| αr15_BaS19r15CI1 | gene | BAbS19_I13490 | R | 1395452 | 1396312 | YP_001935318.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_BaS19r15CI1 | gene | BAbS19_I13500 | R | 1396852 | 1398552 | YP_001935319.1 | cell division protein Fts | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_BaS19r15CI1 | sRNA | BaS19r15CI1 | R | 1396794 | 1396895 | - | sRNA | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_BaS19r15CI1 | gene | BAbS19_I13510 | R | 1398649 | 1399971 | YP_001935320.1 | heat shock protein Hsp7 | Brucella abortus S19 chromosome 1 (NC_010742) |
| αr15_Bm23457r15CI | gene | BMEA_A1472 | R | 1399299 | 1400159 | YP_002733139.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella melitensis ATCC 23457 chromosome I (NC_012441) |
| αr15_Bm23457r15CI | sRNA | Bm23457r15CI | R | 1400641 | 1400742 | - | sRNA | Brucella melitensis ATCC 23457 chromosome I (NC_012441) |
| αr15_Bm23457r15CI | gene | BMEA_A1473 | R | 1400699 | 1402399 | YP_002733140.1 | cell division protein Fts | Brucella melitensis ATCC 23457 chromosome I (NC_012441) |
| αr15_Bm23457r15CI | gene | BMEA_A1474 | R | 1402496 | 1403818 | YP_002733141.1 | cell division protein Fts | Brucella melitensis ATCC 23457 chromosome I (NC_012441) |
| αr15_Bmir15CI1 | gene | BMI_I1436 | R | 1386434 | 1387294 | YP_003107351.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bmir15CI1 | sRNA | Bmir15CI1 | R | 1387776 | 1387877 | - | sRNA | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bmir15CI1 | gene | BMI_I1437 | R | 1387834 | 1389534 | YP_003107352.1 | cell division protein Fts | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bmir15CI1 | gene | BMI_I1438 | R | 1389631 | 1390953 | YP_003107353.1 | cell division protein Fts | Brucella microti CCM 4915 chromosome 1 (NC_013119) |
| αr15_Bs1330r15CI1 | gene | BR1424 | R | 1379039 | 1379899 | NP_698422.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Bs1330r15CI1 | sRNA | Bs1330r15CI1 | R | 1380381 | 1380482 | - | sRNA | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Bs1330r15CI1 | gene | BR1425 | R | 1380439 | 1382139 | NP_698423.1 | cell division protein Fts | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Bs1330r15CI1 | gene | BR1426 | R | 1382236 | 1383558 | NP_698424.1 | cell division protein Fts | Brucella suis 1330 chromosome I (NC_004310) |
| αr15_Ba19941r15CI1 | gene | BruAb1_1419 | R | 1397122 | 1397982 | YP_222110.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Ba19941r15CI1 | sRNA | Ba19941r15CI1 | R | 1398464 | 1398565 | - | sRNA | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Ba19941r15CI1 | gene | BruAb1_1420 | R | 1398522 | 1400222 | YP_222111.1 | cell division protein Fts | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Ba19941r15CI1 | gene | BruAb1_1421 | R | 1400319 | 1401641 | YP_222112.1 | cell division protein Fts | Brucella abortus bv. 1 str. 9-941 chromosome I (NC_006932) |
| αr15_Bmαr15CI1 | gene | BAB1_1443 | R | 1394272 | 1395132 | YP_414815.1 | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Bmαr15CI1 | sRNA | Bmαr15CI1 | R | 1395614 | 1395715 | - | sRNA | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Bmαr15CI1 | gene | BAB1_1444 | R | 1395672 | 1397372 | YP_414816.1 | cell division protein Fts | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Bmαr15CI1 | gene | BAB1_1445 | R | 1397469 | 1398791 | YP_414817.1 | heat shock protein Hsp7 | Brucella melitensis biovar Abortus 2308 chromosome I (NC_007618) |
| αr15_Jspr15C | gene | mma_2445 | R | 2769080 | 2769601 | YP_001354135.1 | phosphinothricin N-acetyltransferas | Janthinobacterium sp. Marseille (NC_009659) |
| αr15_Jspr15C | sRNA | Jspr15C | R | 2769681 | 2769776 | - | sRNA | Janthinobacterium sp. Marseille (NC_009659) |
| αr15_Jspr15C | gene | mma_2446 | R | 2769784 | 2770767 | YP_001354136.1 | tricarboxylate binding receptor | Janthinobacterium sp. Marseille (NC_009659) |
| αr15_Bor15CI1 | gene | BOV_1381 | D | 1387334 | 1387726 | YP_001259317.1 | transposase Orf | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
| αr15_Bor15CI1 | sRNA | Bor15CI1 | R | 1387928 | 1388029 | - | sRNA | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
| αr15_Bor15CI1 | gene | BOV_1382 | R | 1387986 | 1389686 | YP_001259318.1 | cell division protein Fts | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
| αr15_Bor15CI1 | gene | BOV_1383 | R | 1389783 | 1391105 | YP_001259319.1 | cell division protein Fts | Brucella ovis ATCC 25840 chromosome I (NC_009505) |
References
- del Val C, Rivas E, Torres-Quesada O, Toro N, Jiménez-Zurdo JI (December 2007). "Identification of differentially expressed small non-coding RNAs in the legume endosymbiont Sinorhizobium meliloti by comparative genomics". Molecular Microbiology. 66 (5): 1080–91. doi:10.1111/j.1365-2958.2007.05978.x. PMC 2780559. PMID 17971083.
- Ulvé VM, Sevin EW, Chéron A, Barloy-Hubler F (December 2007). "Identification of chromosomal alpha-proteobacterial small RNAs by comparative genome analysis and detection in Sinorhizobium meliloti strain 1021". BMC Genomics. 8 (467): 467. doi:10.1186/1471-2164-8-467. PMC 2245857. PMID 18093320.
- Valverde C, Livny J, Schlüter JP, Reinkensmeier J, Becker A, Parisi G (September 2008). "Prediction of Sinorhizobium meliloti sRNA genes and experimental detection in strain 2011". BMC Genomics. 9 (406): 416. doi:10.1186/1471-2164-9-416. PMC 2573895. PMID 18793445.
- Córdoba JM, Chavarro C, Schlueter JA, Jackson SA, Blair MW (July 2010). "Integration of physical and genetic maps of common bean through BAC-derived microsatellite markers". BMC Genomics. 11 (245): 436. doi:10.1186/1471-2164-11-436. PMC 3091635. PMID 20637113.
- Nawrocki EP, Kolbe DL, Eddy SR (May 2009). "Infernal 1.0: inference of RNA alignments". Bioinformatics. 25 (10): 1335–7. doi:10.1093/bioinformatics/btp157. PMC 2732312. PMID 19307242.
- Will S, Reiche K, Hofacker IL, Stadler PF, Backofen R (2007). "Inferring Noncoding RNA Families and Classes by Means of Genome-Scale Structure-Based Clustering". PLoS Comput Biol. 4 (65): e65. doi:10.1371/journal.pcbi.0030065. PMC 1851984. PMID 17432929.CS1 maint: uses authors parameter (link)
- I. L. Hofacker, W. Fontana, P. F. Stadler, L. S. Bonhoeffer, M. Tacker and P. Schuster (1994). "Fast folding and comparison of RNA secondary structures". Monatshefte für Chemie. 125 (2): 167–188. doi:10.1007/BF00818163.CS1 maint: multiple names: authors list (link)
- Bernhart SH, Hofacker IL, Will S, Gruber AR, Stadler PF (November 2008). "RNAalifold: improved consensus structure prediction for RNA alignments". BMC Bioinformatics. 9 (474): 474. doi:10.1186/1471-2105-9-474. PMC 2621365. PMID 19014431.
- Wilms I, Voss B, Hess WR, Leichert LI, Narberhaus F (April 2011). "Small RNA-mediated control of the Agrobacterium tumefaciens GABA binding protein". Molecular Microbiology. 80 (2): 492–506. doi:10.1111/j.1365-2958.2011.07589.x. PMID 21320185.
- Torres-Quesada O, Oruezabal RI, Peregrina A, Jofre E, Lloret J, Rivilla R, Toro N, Jiménez-Zurdo JI (2010). "The Sinorhizobium meliloti RNA chaperone Hfq influences central carbon metabolism and the symbiotic interaction with alfalfa" (PDF). BMC Microbiol. 6.
- Torres-Quesada O, Millán V, Nisa-Martínez R, Bardou F, Crespi M, Toro N, Jiménez-Zurdo JI (2013-07-15). "Independent activity of the homologous small regulatory RNAs AbcR1 and AbcR2 in the legume symbiont Sinorhizobium meliloti". PLOS One. 8 (7): e68147. doi:10.1371/journal.pone.0068147. PMC 3712013. PMID 23869210.
- MacLellan SR, MacLean AM, Finan TM (June 2006). "Promoter prediction in the rhizobia". Microbiology. 152 (Pt 6): 1751–63. doi:10.1099/mic.0.28743-0. PMID 16735738.
- Novichkov PS, Rodionov DA, Stavrovskaya ED, Novichkova ES, Kazakov AE, Gelfand MS, Arkin AP, Mironov AA, Dubchak I (July 2010). "RegPredict: an integrated system for regulon inference in prokaryotes by comparative genomics approach". Nucleic Acids Research. 38 (Web Server issue): W299–307. doi:10.1093/nar/gkq531. PMC 2896116. PMID 20542910.
- Gama-Castro S, Salgado H, Peralta-Gil M, Santos-Zavaleta A, Muñiz-Rascado L, Solano-Lira H, Jimenez-Jacinto V, Weiss V, García-Sotelo JS, López-Fuentes A, Porrón-Sotelo L, Alquicira-Hernández S, Medina-Rivera A, Martínez-Flores I, Alquicira-Hernández K, Martínez-Adame R, Bonavides-Martínez C, Miranda-Ríos J, Huerta AM, Mendoza-Vargas A, Collado-Torres L, Taboada B, Vega-Alvarado L, Olvera M, Olvera L, Grande R, Morett E, Collado-Vides J (January 2011). "RegulonDB version 7.0: transcriptional regulation of Escherichia coli K-12 integrated within genetic sensory response units (Gensor Units)". Nucleic Acids Research. 39 (Database issue): D98–105. doi:10.1093/nar/gkq1110. PMC 3013702. PMID 21051347.
- Bailey TL, Elkan C (1994). "Fitting a mixture model by expectation maximization to discover motifs in biopolymers". Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. AAAI Press, Menlo Park, California: 28–36.
- Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS (2007). "Quantifying similarity between motifs". Genome Biology. 8 (2): R24. doi:10.1186/gb-2007-8-2-r24. PMC 1852410. PMID 17324271.
- Vinayagam A, del Val C, Schubert F, Eils R, Glatting KH, Suhai S, König R (March 2006). "GOPET: a tool for automated predictions of Gene Ontology terms". BMC Bioinformatics. 7: 161. doi:10.1186/1471-2105-7-161. PMC 1434778. PMID 16549020.
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (September 2005). "Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research". Bioinformatics. 21 (18): 3674–6. doi:10.1093/bioinformatics/bti610. PMID 16081474.
- del Val C, Ernst P, Falkenhahn M, Fladerer C, Glatting KH, Suhai S, Hotz-Wagenblatt A (July 2007). "ProtSweep, 2Dsweep and DomainSweep: protein analysis suite at DKFZ". Nucleic Acids Research. 35 (Web Server issue): W444–50. doi:10.1093/nar/gkm364. PMC 1933246. PMID 17526514.