Étard reaction
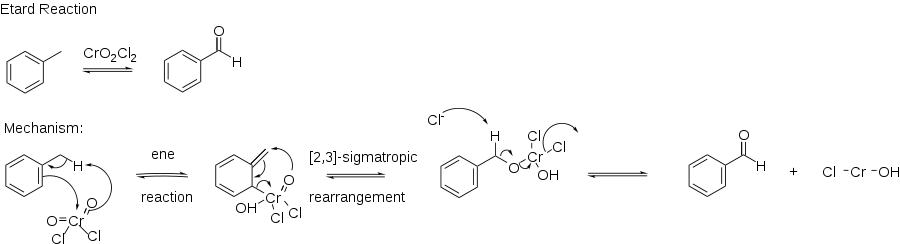
The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride.[1][2][3] For example, toluene can be oxidized to benzaldehyde.
It is named for the French chemist Alexandre Léon Étard (5 January 1852, Alençon – 1 May 1910).
Reaction mechanism
The reaction mechanism proceeds via an ene reaction with chromyl chloride, forming the precipitated Étard complex. The Étard complex is then decomposed by a [2,3] sigmatropic rearrangement under reducing conditions to prevent further oxidation to a carboxylic acid. Reducing conditions for the decomposition of the Étard complex are provided by saturated aqueous sodium sulphite. Typical solvents for the reaction include carbon disulfide, dichloromethane[4] chloroform, and carbon tetrachloride, with carbon tetrachloride being the most common. To obtain a highly purified aldehyde product, the Étard complex precipitate is often purified before decomposition in order to prevent reaction with any unreacted reagent. The reaction is normally carried out for a few days to several weeks and the yields are high.[5][6]
Limitations
The Étard reaction is most commonly used as a relatively easy method of converting toluene into benzaldehyde. Obtaining specific aldehyde products from reagents other than toluene tends to be difficult due to rearrangements. For example, n-propylbenzene is oxidized to propiophenone, benzyl methyl ketone, and several chlorinated products, with benzyl methyl ketone being the major product.[7][8] Another example arises from the Étard reaction of trans-decalin which results in a mixture of trans-9-decalol, spiro [4.5]decan-6-one, trans-1-decalone, cis-1-decalone, 9,10-octal-1-one, and 1-tetralone.[9]
Other oxidation reagents like potassium permanganate or potassium dichromate oxidize to the more stable carboxylic acids.
Uses
Oxidation of toluene to benzaldehyde is quite a useful conversion. Benzaldehyde is routinely used for its almond flavor. The aldehyde is comparatively reactive and readily participates in aldol condensations. Benzaldehyde can serve as a precursor for various compounds, including dyes, perfumes, and pharmaceuticals. For example, the first step in the synthesis of ephedrine is condensation of benzaldehyde with nitroethane . Additionally, benzaldehyde is instrumental in the synthesis of phentermine.[10] Unlike other oxidising agents (like KMnO4 or CrO3 etc.), chromyl chloride does not oxidise aldehyde to carboxylic acid.
References
- Étard, A. (1880). "Sur la synthèse desaldéhydes aromatiques; essence de cumin" [On the synthesis of aromatic aldehydes ; essence of cumin]. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences (in French). 90: 534. Archived from the original on 1 March 2012.
- Étard, A. (1881). "Recherches sur le rôle oxydant de l'acide chlorochromique". Annales de Chimie et de Physique (in French). 22: 218–286. Archived from the original on 1 March 2012.
- Hartford, W. H. & Darrin, M. (1958). "The Chemistry Of Chromyl Compounds". Chemical Reviews. 58: 1–61. doi:10.1021/cr50019a001.
- F. Freeman (2004). "Chromyl Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc177..}}
- Necsoiu, I.; Balaban, A. T.; Pascaru, I.; Sliam, E.; Elian, M.; Nenitzescu, C. D. (1963). "The mechanism of the Étard reaction". Tetrahedron. 19 (7): 1133–1142. doi:10.1016/s0040-4020(01)98572-2.
- Wheeler, Owen H. (1958). "Étard Reaction: I. Its Scope and Limitation with Substituted Toluenes". Canadian Journal of Chemistry. 36 (4): 667–670. doi:10.1139/v58-093.
- Renţea, C. N.; Necşoiu, I.; Renţes, M.; Ghenciulescu, A. & Nenitzescu, C. D. (1966). "Étard reaction—III: Oxidation of N-propylbenzene and methylcyclohexane with chromyl chloride". Tetrahedron. 22 (10): 3501–3513. doi:10.1016/s0040-4020(01)92538-4.
- Wiberg, K. B.; Marshall, B. & Foster, G. (1962). "Some observations on the Étard reaction". Tetrahedron Letters. 3 (8): 345–348. doi:10.1016/s0040-4039(00)70878-1.
- Renţea, C. N.; Renţea, M.; Necşoiu, I. & Nenitzescu, C. D. (1968). "Étard reaction—VI: Oxidation of cis and trans-decaline with chromyl chloride". Tetrahedron. 24 (13): 4667–4676. doi:10.1016/s0040-4020(01)98663-6.
- Vardanyan, Ruben S. & Hruby, Victor J. (2006). Synthesis of Essential Drugs (first ed.). Amsterdam: Elsevier Science. ISBN 978-0-444-52166-8.