Golgi bodies
Golgi bodies, the Golgi complex or the Golgi apparatus, named after the Italian biologist Camillo Golgi, are composed of numerous sets of smooth cisternae, which are coated with lipid membranes. Each disc-shaped cisternae forms a structure that resembles a stack of plates, called a Golgi stack. The Golgi complex contains a great number of vesicles. These vesicles are used to send molecules to the cellular membrane, where they are excreted. There are also larger secretory vesicles, which are used for selective excretion.

| Live, reproduce, die Biology |
| Life as we know it |
|
| Divide and multiply |
| Greatest Great Apes |
|
v - t - e |
This article is only a brief description of the subject and is not intended to give a full explanation.
Check out the "see also" or "references" sections, or Wikipedia's article for more detail.
The Golgi is principally responsible for directing molecular traffic in the cell - nearly all molecules pass through the Golgi complex at some point in their existence. The sorting is mediated by the vesicles. When proteins bind with their appropriate receptor on the vesicle, they are encoated in the vesicle and transported away.
The Functions of the Golgi Complex
What types of secretion are controlled by the Golgi complex?
The Golgi complex controls trafficking of different types of proteins. Some are destined for secretion. Others are destined for the extracellular matrix. Finally, other proteins, such as lysosomal enzymes, may need to be sorted and sequestered from the remaining constituents because of their potential destructive effects. This figure shows the two types of secretory pathways. The regulated secretory pathway, as its name implies, is a pathway for proteins that requires a stimulus or trigger to elicit secretion. Some stimuli regulate synthesis of the protein as well as its release. The constitutive pathway allows for secretion of proteins that are needed outside the cell, like in the extracellular matrix. It does not require stimuli, although growth factors may enhance the process.
Golgi complex regulation of insertion of plasma membrane proteins
Plasma membrane proteins are inserted in the membrane at the level of the rough endoplasmic reticulum. The protein sequence is coded for membrane insert start and stop sites. This directed the insertion and alignment points. Those that are multipass proteins have multiple start and stop sites.
The important role of the Golgi Complex is to make certain the plasma membrane proteins reach their destination.
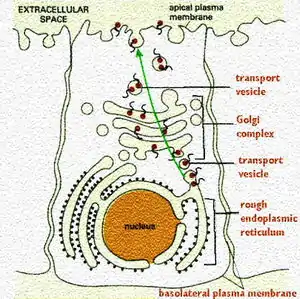
This figure shows the route. Note that the orientation of the protein is maintained so that the region destined to project outside the cell (a receptor binding site, for example), ends up in that place. In order to do this, it must be placed so that it faces inside the vesicle.
Golgi complex adding carbohydrate groups to a glycoprotein
The Golgi complex is compartmentalized. Phosphorylation occurs in the Cis region. In other regions, different types of carbohydrates are added as a glycoprotein passes through the cisternae. This figure illustrates the different regions where sugars like mannose (man), galactose (gal), etc are added. The final sorting is done in the Trans Golgi complex.
The functional differentiation of the Golgi complex can be studied with the electron microscope with specific techniques that detect enzymes. The cis region is rich in lipid-bearing membranes and can be delineated by osmium tetroxide labeling. The middle regions label for enzymes that add carbohydrates or other groups on the product. The inner, or Trans region, is the area where the lysosomes are sorted. Therefore, it is heavily labeled for acid phosphatase.
There is much interest in understanding how the different Golgi cisternae are organized and differentiated. A number of models exist, however a favorite is called the "Maturational model" [1]
The Maturational model suggests that the new vesicles from the ER enter the cis Golgi network and retrograde vesicles (bearing COPI) coats move to merge with the cis region cisternae. These carry Golgi complex processing enzymes and their targeting to this region may be dependent on the low concentration of these processing enzymes. Then, as processing continues, the middle cisternae contain more mature product and lower amounts of the enzymes needed in the beginning. Finally, the trans region is specialized for sorting, containing receptors to sort and isolate lysosomal enzymes, for example.[2]
Protein transport to the rough endoplasmic reticulum
Sometimes vital proteins needed in the rough endoplasmic reticulum are transported along with the other proteins in the Golgi complex.[3] The Golgi complex has a mechanism for trapping them and sending them back to the rough endoplasmic reticulum.

This cartoon shows the process. The protein destined for secretion is red. The blue protein must remain in the rough endoplasmic reticulum. The rough endoplasmic reticulum has inserted a receptor protein on the membrane it sends to the Golgi complex in the transitional vesicles (shown in green). These are retrograde vesicles and are therefore coated with "COPI" (coatamer). The ER protein receptor captures all of the protein that carries the ER residency signal. . Vesicles then bud from the Golgi complex and move back to the rough endoplasmic reticulum. The receptor can circulate and continue to return the proteins needed by the endoplasmic reticulum.
Golgi bodies are known as the mailroom of the cell. Golgi bodies identify any substance and delievers it to the part of the cell that needs it the most. They receive those materials from the endoplasmic reticulum.
See also
References
- Bannykh S.I. and Bakch, W.E. Membrane Dynamics at the Endoplasmic Reticulum Golgi Interface J Cell Biol 138: 1-4 1997)
- Wooding, S and H.R.B. Pelham, The dynamics of Golgi protein traffic visualized in living yeast cells. Molecular biology of the cell. 9: 2667-2680 1998
- Cole, N.B., Ellenberg, J, Song, J, DiEuliis, D and Lippincott-Schwartz, J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol 1-15, 1998.