FAQ on radioactivity and nuclear technology
Radioactivity and nuclear technology are a common subject of myths, urban legends and misinformation. Many of them lead to an exaggerated sense of danger and irrational fear of anything nuclear, leading to faulty perceptions of risk and preventing societies from taking full advantage of these technologies. In a humble attempt to set the record straight, this article provides answers to some important questions on this subject.
| Splitting more than hairs Nuclear energy |
| Ionizing pages |
v - t - e |
Radioactivity
What is radioactivity?
Radioactivity is the spontaneous disintegration of certain kinds of atomic nuclei. Over time, these nuclei will emit heat and highly energetic particles known as ionizing radiation, then form other nuclei known as decay products. This process is called radioactive decay. It is impossible to predict when a particular nucleus will disintegrate, but when observing a large number belonging to the same nuclide (kind of nucleus), the number of intact ones will decrease exponentially. Different radioactive nuclides have different rates of decay. These rates cannot be influenced in any significant way.[1] This fact is the basis of radiometric dating, which is very important in geology.
Radioactivity is a manifestation of quantum tunneling.
Is radioactivity natural?
Yes. Radium is a naturally occurring, intensely radioactive element. Uranium that fuels nuclear power plants is also mined from natural deposits. There are many sources of background radiation; among them, natural sources are responsible for a large majority of radiation absorbed by humans. The man-made portion is dominated by medical exposures.[2]
Nuclear reactors are also found in nature. The Sun generates its energy from fusion, so it is a natural fusion reactor. In 1972, the remnants of a natural fission reactor were found in a deposit of uranium ore in Oklo, Gabon.[3]
Note that considering natural things "good" and artificial things "bad" without any other evidence is generally wrong and known as appeal to nature.
What is ionizing radiation?
Radiation means any stream of elementary particles. For example, visible light is radiation consisting of photons with wavelengths between 390 and 700 nanometers, and beta radiation is radiation consisting of electrons. It is useful to conceptualize different kind of radiation as different "colors of light" and different materials as transparent to different "colors". For example, wood is opaque to visible light, but somewhat transparent to gamma radiation. Zirconium is likewise opaque to visible light, but very transparent to neutrons.
Ionizing radiation is any radiation composed of particles which have enough energy to create ion pairs in the air, making it conductive to electric current. Detecting the ionization of a suitably chosen gas is a common method of detecting ionizing radiation; indeed, this is how a Geiger-Müller counter works.
What kinds of nuclear radiation are there?
There are three common kinds of ionizing radiation produced by nuclear processes, though this is list is not exhaustive:
- Alpha radiation consists of helium-4 nuclei (two protons and two neutrons). It is emitted in the radioactive decay of the heaviest elements, such as uranium or thorium. It is highly damaging to living organisms, but has very short range and cannot penetrate the skin. This means it is only dangerous when an alpha-radioactive substance is eaten or inhaled.
- Beta radiation consists of fast-moving electrons or positrons. It is emitted from nuclides which have too many neutrons, such as strontium-90. It travels a few meters in the air and cannot penetrate the skin or thin metal shields. A beta ray can excite secondary gamma radiation when it strikes an object.
- Gamma radiation consists of very high energy photons. It is emitted from nuclei which just underwent decay and are in an excited state. Gamma radiation is the most penetrating. It can travel many meters in the air and is only stopped by thick shielding. (A few meters of water is enough.)
There are many other less common kinds of ionizing radiation, since a fast enough stream of almost any kind of elementary particles will be ionizing. This includes positron radiation and proton radiation, for example.
In addition, there are two other kinds of ionizing radiation you might run into, which are generally produced by processes that aren't nuclear:
- X-ray radiation consists of photons with an energy lower than gamma radiation. It is generated in processes not involving radioactivity in specially designed electron lamps and synchrotrons.
File:Wikipedia's W.svg - Ultraviolet light consists of photons with an energy lower than X-ray radiation, but still higher than visible light. The upper frequency range of the ultraviolet spectrum ("hard" ultraviolet) is energetic enough to be ionizing. The lower frequency range, such as the UV-A you get in a tanning salon or the UV-B that gives you sunburn, isn't ionizing.
One other kind of nuclear radiation bears mentioning:
- Neutron radiation consists of neutrons, the neutral particles making up the nuclei of atoms. It is emitted as a result of several nuclear reactions, most importantly fission.
Do things subjected to radiation become radioactive?
Generally, no. When an object is irradiated with alpha, beta or gamma radiation, there are some chemical changes, but it does not become radioactive. Ionizing radiation is a lot like visible light: no matter how long you sunbathe, you won't start glowing in the dark. Many people are afraid of food irradiation (which uses ionizing radiation) — or even microwave ovens (which don't) — because they do not understand this simple concept.
The exception to the above rule is neutron radiation, found mainly inside working reactors, which can make most substances radioactive. Although in most cases this is not a desirable thing, it can also be very useful. The activated substances can be detected and identified using a suitable radiation detector, and therefore the chemical composition of the sample can be determined without destroying it. This is mainly done at research reactors.
Why are nuclear workers tested for radiation?
They are not tested for irradiation, but for contamination - whether they have radioactive substances on their skin or clothes. Contamination does not indicate that new radioactivity is created, but that some of it is misplaced. When somebody tests positive, they must wash thoroughly and change their clothes. In cases where radioactive substances have been absorbed into the body more extensive treatment may be required.
In more familiar terms, the difference between irradiation and contamination is like the difference between smelling cow dung and having it on your shoes. When you are being irradiated (smelling dung), the exposure ceases when you leave the high radiation area (move away from the cow pie). When you are contaminated (dirty with dung), you will receive more and more radiation from the decaying radioactive substances until you wash them off (clean your shoes).
Do radioactive things glow in the dark?
Generally, no. However, they will glow in the dark when mixed with a substance that emits visible light when struck by ionizing radiation.[4] The light emitting substance in some watch dials is actually a non-radioactive phosphor excited by a small amount of radioactive material to emit light. Nowadays luminous dials on high quality watches use tritium as the radioactive material, while radium was used in the past.
There are two further circumstances when highly radioactive objects can glow. The first one is when they are submerged in water, when Cherenkov radiation — arising from particles traveling faster than the speed of light in water — will appear as a blue light. This is most often seen in photos of spent fuel pools and research reactors. The second case is when an object is so intensely radioactive that it spontaneously heats up to thousands of degrees - it will emit a red or yellow thermal glow. This happens e.g. for fresh plutonum-238 pellets, used as a power source for deep space probes.
What is nuclear fission?
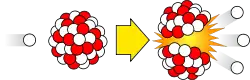
Nuclear fission is the disintegration of certain atomic nuclei when hit by a neutron. After absorbing the neutron, these nuclei become highly unstable and shatter into pieces, releasing a large amount of thermal energy and more neutrons along the way. Because this process requires neutrons, its rate can be controlled, as opposed to radioactive decay.
Nuclides which can undergo fission are called fissile.
What is a chain reaction?
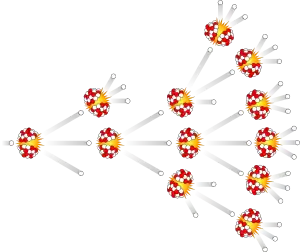
Each event of nuclear fission generates more than one secondary neutron. When enough fissile material is concentrated in one place, the neutrons created in the first fission reaction will strike other fissile nuclei, causing further fissions releasing more neutrons and so on. In this way, a substantial amount of the material can be fissioned to release a tremendous amount of heat.
Relevant video illustrating the concept.
When a nuclear chain reaction is engineered to consume a lot of fissile material in a very short time, it results in an extremely powerful explosion. This fact can be exploited to construct nuclear bombs. When the reaction is engineered to be self-limiting, a slowly fissioning block of fissile material can be used as an abundant source of heat, which can then be converted to electricity. This is known as nuclear power. While the underlying process is the same, these two uses require very different materials and conditions, so the relation between them is about the same as between napalm and tealights. See also this question.
What is nuclear fusion?
Nuclear fusion is the synthesis of heavier elements from lighter ones, accompanied by a release of heat and energetic particles. This process is the energy source of all bright stars. In the Sun, the energy comes from fusion of hydrogen into helium. On Earth, the most promising source of fusion energy is the reaction of deuterium and tritium, isotopes of hydrogen, which yields helium and a neutron.
Fusion requires conditions of extreme temperature and pressure, and as such is not expected to be a practical energy source on Earth for several decades. However, such conditions can easily be generated during a fission-based nuclear explosion. This fact can be (and has been) exploited to build more powerful nuclear bombs.
What are the units for measuring radiation?
There are three basic units:
- The becquerel (Bq) is a measure of activity, or the frequency of disintegrations in a given sample. 1 Bq is equal to 1 disintegration per second. It is an extremely small unit, often used with SI prefixes. The human body normally has a specific activity of 100 Bq/kg, which translates to 7000 Bq total activity for an average adult human. Activity only specifies, in an indirect way, the amount of radioactive substance. It says little about its health or environmental impact.
- The gray (Gy) is a measure of absorbed dose, equivalent to J/kg. It specifies how much energy per unit mass was absorbed by a given object, organism or tissue. This quantity is directly measurable. Grays are used in areas of nuclear technology which do not involve living things, and also sometimes in radiation biology research.
- The sievert (Sv) is a measure of the biological effect of the dose, called the equivalent dose.
File:Wikipedia's W.svg The equivalent dose cannot be measured directly. Its precise value is computed in a fairly complex way from the absorbed dose, with various weights depending on the irradiated organs, the age of the person, the type of radiation, its energy, etc. However, in many situations this calculation can be greatly simplified. For gamma and beta radiation received by adults uniformly over the whole body, the absorbed dose in Gy is equal to the dose equivalent in Sv.
When a radioactive nuclide is eaten or inhaled, the biological effect can be computed by multiplying the activity of the ingested sample by the dose coefficient of that nuclide, usually given in Sv/Bq. These coefficients are available from radiation protection authorities.
It is worth clearing up one misconception: when a dose is given for a particular group of people, e.g. infants, the radiosensitivity of that group is already taken into account. A baby receiving 100 mSv will experience the same effects as an adult receiving 100 mSv. Of course these two dose equivalents do not correspond to the same absorbed dose in grays.
Additional units include:
- The rad and rem are obsolete units still used in the U.S. 1 gray is equal to 100 rad and 1 sievert is equal to 100 rem. Rem is an acronym for Röntgen-Equivalent Man. Rad is not an acronym, nor is it what the kids say when they see something totally radical.
- The curie (Ci) is another obsolete unit, equal to 3.7 × 1010 becquerel. It is still occasionally used to specify a quantity of a radioactive substance, e.g. ordering 1 curie of cesium-137 from the warehouse would get you about 10 milligrams of cesium-137.
- The röntgen (R) (or roentgen) is a measure of air ionization equal to 2.58×10−4 C/kg. It is used to measure ambient radiation levels in the post-Soviet states and on some dosimeters. For common energies of X-ray and gamma radiation, a radiation field causing 1 roentgen of ionization in air will deposit roughly one rad (0.96 rads to be exact) into soft tissue, which is in turn roughly equivalent to 1 rem for whole body irradiation. In other words, 1 R/h ≈ 0.01 Sv/h = 10 mSv/h.
- Times background is sometimes used as an informal unit of dose rate. One "times background" is usually 3 mSv/year.
- Counts per second (cps) is the unit of raw measurement given by radiation detectors. One count corresponds to one particle interacting with the detector. It is often used in places where only the relative strengths of radiation fields need to be compared. A single measurement in this unit is meaningless without knowing the calibration curve for the given detector.
What are the possible effects of radiation exposure?
There are three types of possible effects.
Deterministic effects always happen when a particular threshold dose is exceeded, increase in severity for higher doses, and are absent for lower doses. Deterministic effects start above 250 mSv - a very high dose which is only experienced by radiation therapy patients and may be experienced by some emergency response workers during a nuclear accident. Below is a table of acute whole body doses (delivered in a short time to the entire body) and the deterministic effects they cause.
When the dose is fractioned, i.e. delivered in batches with a pause of several hours between each exposure, the effect is significantly weaker than when the same dose is delivered all at once. Doses delivered in a short time have more severe effects than those delivered over a long time. This also applies to stochastic effects to some extent.
| Dose | Deterministic effect |
|---|---|
| below 250 mSv | No effect |
| 250 mSv | Lowered blood cell count |
| 1000 mSv | Mild radiation poisoning: low white blood cell count, fatigue, weakness |
| 2000 mSv | Moderate radiation poisoning: vomiting, diarrhea, headache, fever |
| 3000 mSv | Loss of hair |
| 3000 mSv | 50% chance of death from radiation poisoning (without medical care) |
| 6000 mSv | Heavy radiation poisoning: vomiting, diarrhea, high fever, dizziness, hypotension, death very likely |
| 8000 mSv | Certain death, rapid incapacitation |
| 30000 mSv | Certain death in 48 hours |
| 500-2000 mSv to lens | Clouding of eye lens |
| 5000 mSv to lens | Cataract |
| 2500-6000 mSv to ovary | Permanent infertility |
| 3500-6000 mSv to testicles | Permanent infertility |
Stochastic effects happen with some probability dependent on the dose you absorb. They can only be attributed to radiation by investigated groups of people with similar exposures. The most important stochastic effect is cancer. The risk of dying from radiation-induced cancer is 5.5% per sievert, or 0.5% per 100 mSv. It is dubious that this linear relationship extends to low doses (below 100 mSv), but this is a common assumption in radiation protection. See linear no-threshold. It is also known from animal studies that radiation exposure may lead to an increase in birth defects in offspring. However, increased birth defects were not found in populations affected by the atomic bombings of Japan[5] or the Chernobyl accident.[6]
Psychological effects arise from the fear of radiation and evacuations following a radioactive release. These might include post-traumatic stress disorder, depression, alcoholism, cardiovascular problems, irresponsible sexual behavior and suicide. In the aftermath of past nuclear accidents such as Chernobyl, these effects were many times worse than either the deterministic or stochastic effects. They would also be the most important after the detonation of a dirty bomb.[7] Psychological effects are of course highly variable depending on the person's level of fear. As such, there is a case to be made that the most harmful effects of nuclear accidents are partially caused by the activities of the anti-nuclear movement.
What are the limits for radiation exposure?
The internationally recognized limits for acceptable exposures caused by nuclear installations are:[8][9]
- 1 mSv/year for members of the public
- 20 mSv/year for nuclear workers
- 250 mSv total for life-saving emergency response
There are no limits for medical exposures or natural radiation. Typical exposure from these is 3 mSv/year.
Does radiation create dangerous mutants?
There is a common science fiction trope of radiation turning someone / something into a mutant. As detailed above, when increasing the dose of radiation, an organism will first experience no apparent ill effects, then exhibit stunted growth and generally deteriorating health, and finally die. If it survives, it will have a higher risk of developing cancer, it might be sterile or its offspring might have more birth defects. The irradiated organism will never undergo any dramatic changes in appearance, because the DNA damage created by irradiation is completely random and not identical in all the cells. Fictional "mutations", such as grotesquely increased size, extra limbs, superior strength or a green glow, are nothing more than a fantasy.
Mutations are undesirable when it comes to humans, but they may be useful when done to plants. Radiation-induced mutations are an important tool in plant breeding.[10]
Does radiation cause birth defects?
The Internet has an abundant supply of shock pictures described as "mutated babies" which are usually described as a product of "Chernobyl radiation" or "depleted uranium bombs". These pictures practically never match their description, and most of them depict birth defects not caused by radiation. The phrase "depleted uranium bombs" should already trigger bullshit alarms, since depleted uranium is used in armor piercing ammunition, not bombs.
The human studies conducted so far, primarily on the survivors of atomic bombings of Japan[5] and the Chernobyl accident,[6] lead to the following conclusions:
- Radiation exposure in utero, i.e. in the mother's womb, has different effects depending on the age of the fetus. Before 2-3 weeks, it increases the incidence of miscarriage, but not birth defects. Between 4 and 11 weeks, it increases the incidence of malformations and stillbirth. Between 11 and 15 weeks, it may cause mental deficiencies and microcephaly. After 20 weeks, the fetus is more resistant to radiation. There is also an elevated but low risk of leukemia.[11]
- While in principle radiation can cause heritable genetic damage, it was not observed in the survivors of atomic bombings or nuclear accidents.[12]
Nuclear power
How does a nuclear reactor work?
Let's describe the most common kind of reactors, the pressurized light water reactor. Several tons of uranium oxide are fashioned into fuel bundles and placed inside a large steel tank (the pressure vessel). The bundles inside the tank form the reactor core. The uranium in the uranium oxide consists of between 0.7% to 5% of uranium-235, which can undergo fission; the rest is uranium-238, which is inert. The core also contains a substance that slows down neutrons, called a moderator. Slow neutrons (also known as thermal neutrons) react much more readily with uranium-235 than do fast neutrons generated by fission. A neutron source is used to start the chain reaction. Fission of uranium-235 in the core generates tremendous amounts of heat, which for a large reactor is equivalent to 1.5 million electric kettles.[13] This heat is removed using a coolant, typically water. The heated coolant is used to generate electricity and returned to the core. Waste heat generated in the process is rejected to a nearby body of water or carried away by steam emitted from cooling towers. The chain reaction is controlled by inserting rods made from neutron-absorbing material, called control rods, into the core. They prevent some neutrons from propagating the chain reaction, thereby slowing it down. To shut down the reactor, all control rods are fully inserted, stopping the chain reaction completely. The decay of fission products continues to produce substantial heat for some time, so the reactor must remain adequately cooled after shutdown.
In most reactors, the chain reaction is naturally self-limiting. The easiest way to do this is to use water as both the moderator and the coolant. When the reactor core gets too hot, some of the water will turn into steam, which is a much worse moderator than liquid water. This causes the neutrons to slow down less, reducing the effectiveness of fission and therefore heat production, leading the reactor to cool down.
As fission progresses in the reactor, the concentration of uranium-235 falls, while the amount of highly radioactive fission products increases. Additionally, a small amount of uranium-238 is converted into plutonium. Fission products are far more radioactive than the fresh fuel bundles — the latter can be handled with gloves. When the concentration of uranium can no longer sustain a chain reaction, part of the core is replaced with fresh fuel. The old, extremely radioactive fuel is moved to a spent fuel pool.
How safe are nuclear reactors?
First we have to pick some objective measure of safety. For example, we might interpret safety narrowly as the frequency of workplace accidents. Here is the frequency of OSHA recordable accidents per 200,000 working hours (100 full-time workers employed for one year) for various United States industries.[14] Surprisingly, nuclear power plants fare better than financial institutions.
| Industry | Accident rate |
|---|---|
| Manufacturing | 5.6 |
| Construction | 5.4 |
| Education | 2.4 |
| Journalism & Broadcasting | 2.0 |
| Financial services | 1.4 |
| Independent artists, writers, and performers | 1.0 |
| Nuclear power | 0.9 |
Another measure, more relevant to the average citizen, is the number of deaths per unit of energy produced, for example deaths per petawatt-hour (one petawatt-hour = one trillion kilowatt-hours). These estimates include the effects of nuclear accidents, dam failures, mine explosions, air pollution and so on. Here is the breakdown:[15]
| Energy source | Deaths/PWh |
|---|---|
| Coal (world, all uses) | 100,000 |
| Coal (world, electricity only) | 60,000 |
| Coal (China, all uses) | 170,000 |
| Coal (USA, all uses) | 15,000 |
| Oil | 36,000 |
| Natural gas | 4,000 |
| Biomass | 12,000 |
| Peat | 12,000 |
| Solar PV (rooftop) | 440 |
| Wind | 150 |
| Hydro (world) | 1,400 |
| Hydro (Europe) | 100 |
| Nuclear | 40 |
According to this measure, nuclear power is the safest energy source, with wind, solar and hydroelectric power close behind. Other analyses of death rates come to similar conclusions, with nuclear faring best or second-best.[16][17][18]
Another possibility is to use the concept of external costs, i.e. costs borne by the society in the form of environmental remediation, days of work lost due to illness, and so on. This is a measure of all unfavorable effects of a given technology on the society, rather than strictly a measure of safety. An extensive study of external costs of energy in Europe, called ExternE, published a report of its conclusions in 2006. The results are summarized below. Note that the results for nuclear assume a discount rate of 0%, which is impossible and unfavorable; results for realistic discount rates would be lower.[19]
All values are in eurocents per kWh.
| Country | Coal | Peat | Oil | Gas | Nuclear | Biomass | Hydro | Solar PV | Wind |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 1–3 | 2–3 | 0.1 | ||||||
| Belgium | 4–15 | 1–2 | 0.5 | ||||||
| Denmark | 4–7 | 2–3 | 1 | 0.1 | |||||
| Finland | 2–4 | 2–5 | 1 | ||||||
| France | 7–10 | 8–11 | 2–4 | 0.3 | 1 | 1 | |||
| Germany | 3–6 | 5–8 | 1–2 | 0.5 | 3 | 0.6 | 0.05 | ||
| Greece | 5–8 | 3–5 | 1 | 0–0.8 | 1 | 0.25 | |||
| Ireland | 6–8 | 3–4 | |||||||
| Italy | 3–6 | 2–3 | 0.3 | ||||||
| Netherlands | 3–4 | 1–2 | 0.7 | 0.5 | |||||
| Norway | 1–2 | 0.2 | 0.2 | 0–0.25 | |||||
| Portugal | 4–7 | 1–2 | 1–2 | 0.03 | |||||
| Spain | 5–8 | 1–2 | 3–5 | 0.2 | |||||
| Sweden | 2-4 | 0.3 | 0–0.7 | ||||||
| United Kingdom | 4–7 | 3–5 | 1–2 | 0.25 | 1 | 0.15 |
From this table we can conclude that nuclear has external costs lower than all fossil fuel technologies, comparable to hydro and solar PV, and higher than wind. The external costs for nuclear are the highest in the Netherlands, which have only one small reactor.
What are the safety mechanisms in a reactor?
Several mechanisms ensure the safe operation of a reactor and prevent the release of radioactive substances into the environment. These can be divided into:
- Active safety - requires the reactor's control systems or the operator to take an action when an abnormal event occurs. This includes emergency shutdown controlled by automatic sensors and multiple backup generators to provide emergency power.
- Passive safety - does not require any action from the control system or an operator, and relies only on the laws of physics to ensure safety during abnormal events. For example, control rods must be actively held out of the reactor by electromagnets, and fall into the core by gravity and/or are inserted by springs when power fails.
New reactor designs, such as AP1000,
There are at least five levels of passive safety in most reactors:
- The uranium oxide fuel itself is resistant to high temperatures and can withstand some overheating while retaining fission products.
- The fuel is put inside a cladding transparent to neutrons, usually an alloy of zirconium. The cladding is water-tight and provides a further barrier.
- The reactor vessel itself provides another layer of contaiment.
- The reactor is placed in a large concrete bunker called the containment building, which is designed to keep the radioactivity inside and protect the reactor from external threats, such as an aircraft impact or a rocket attack.
- The reactor building is located in a sparsely populated area far from major cities to minimize the health impact of a radioactive release.
This approach of multiple independent barriers is called defence in depth.[20]
Are nuclear power plants vulnerable to terrorist attack?
A nuclear power plant is considered a "hard" terrorist target; an attack would be very unlikely to succeed. A Western-type reactor is housed inside a reinforced concrete bunker which can withstand rocket attacks and some airplane crashes.[21][22] However, older reactors tend to have less thick reinforcement and some may not withstand a head on crash of an Antonov
So far there have been no real terrorist attacks that targeted an operating nuclear power plant. There was however a rocket attack against a breeder reactor under construction, perpetrated by an anti-nuclear activist.[25] Explosives without a detonator were detected in a forklift truck at the Ringhals nuclear power plant in Sweden; the perpetrator was not identified.[26] Some plants have been intruded by anti-nuclear protesters.[27][28] None of them managed to reach safety-critical areas of the plant, such as the control room or the inside of the reactor containment building.
There are far more attractive terrorist targets, for instance mass transit systems, chemical plants, oil and gas pipelines, LNG facilities and dams. Attacks on these would have a higher chance of success and higher potential impact.[29]
Can a nuclear reactor explode like a nuclear bomb?
No, it can't. The fissile material in a reactor is not concentrated enough to create a bomb-like explosion, even if all safety mechanisms were to fail. A runaway chain reaction would at most blow the reactor vessel apart with steam pressure and contaminate the containment building.[30]
How much nuclear waste is produced?
A large, one-gigawatt nuclear reactor will generate 200-350 m3 of low- and intermediate-level nuclear waste and 27 tons (20 m3) of high-level waste (spent fuel) each year, which will take up 75 m3 if put into disposal containers without further processing. If the fuel is reprocessed, the end product will be 3 m3 of fission products immobilized in glass, which will take up 28 m3 after putting it in disposal containers. A coal power plant of the same power will produce 400,000 tons of fly ash in the same period,[31] which actually contains a similar amount of radioactive waste as the nuclear plant, due to the trace amounts of uranium and thorium in coal.[32]
To compare the high-level waste figures against other waste, we will use the UK as an example of the average industrialized country. If the UK used only nuclear electricity, the average resident's lifetime share of high-level waste would take up the volume of one milk carton (1.12 litres), and the volume of two large soda bottles when placed in containers (4.21 litres). If the waste was reprocessed, it would fill less than a coffee cup (152 ml), and two wine bottles when put in containers (1.57 litres).[33] This compares rather favorably with statistics on ordinary waste in the UK, where 517 kg of municipal waste and 83 kg of hazardous waste per person is produced every year, or 41411 kg and 6648 kg per person per average life expectancy, respectively.[34]
What happens with spent fuel?
After being discharged from the reactor, spent fuel is first put in a spent fuel pool, where it is continually cooled for a few years. When the fuel bundles no longer generate enough heat to melt themselves, they can be put in reinforced concrete casks, which is called dry storage. After cooling down, the fuel optionally undergoes nuclear reprocessing
Right now, there are no repositories for spent fuel in operation. The largest progress toward creating one was made in Sweden and Finland, where applications were submitted to national nuclear regulators. There is also an operating military waste repository in the U.S., the Waste Isolation Pilot Plant.
What does nuclear waste look like?
The spent fuel storage pool looks like this:
Spent fuel in dry storage casks looks like this:
See here for a photo of a facility that stores 30 years' worth of spent fuel from the decommissioned Connecticut Yankee nuclear power plant.
The stereotypical image of nuclear waste is a glowing green fluid kept in rusting barrels at a landfill. This is a misconception, and likely derived from the image of toxic chemical waste as publicized in the late '70s by places such as the Valley of the Drums
The closest thing to the stereotypical image is the storage of depleted uranium in the form of uranium hexafluoride, a byproduct of uranium enrichment, in large steel cylinders. This is because the stuff is basically chemical waste and only weakly radioactive. It will be eventually converted to uranium dioxide, which is a far safer form of storage, although progress on this has been slow. Depleted uranium could be used in the future as fuel for breeder reactors, so the stuff is not completely worthless. The cylinders look like in the photo below, with the second picture showing humans for scale:


In addition to spent fuel, nuclear power generates intermediate and low level waste. Intermediate level waste is considerably less dangerous than spent fuel, and can be stored in steel barrels filled with concrete, which will eventually also be placed in a deep geological repository.[35] Low level waste poses a minor hazard and is stored in near-surface repositories, typically in concrete-lined pits.[36]
How long will nuclear waste be dangerous?
Nuclear waste is no longer considered dangerous when its radioactivity drops below some threshold. Since the waste should eventually be buried underground, it seems reasonable to pick the radioactivity level of uranium ore from which it was produced as the threshold - people have lived near uranium deposits for centuries without many problems. With these assumptions:[37]
- Without reprocessing, the waste will be dangerous for 20,000 years.
- With reprocessing, the separated fission products will be dangerous for 300 years (10 half-lives of strontium-90 and cesium-137, the longest-lived fission products).
Will deep geological repositories be safe?
Yes, it is almost 100% certain, as long as the repository is properly designed.
The primary way in which a repository can fail and create a danger is if it leaks a lot of radioactive substances into the groundwater, which can then enter the environment in sufficient quantities to cause damage. This risk is extremely small, as the repositories are deep underground, store the nuclear waste in an insoluble form, and protect it from water with several layers of material. The site is chosen in such a way that the geology of the area will naturally limit the spread of radioactivity should the integrity of the repository be compromised. The reliability of deep geological storage over extremely long timescales was already proven in nature. 2 billion years ago, there was a natural nuclear reactor operating in a uranium deposit in Gabon. When it was discovered in 1972, its waste products were still in place.[38]
Even if the entire contents of the repository were to somehow magically appear on the surface one day, it would not cause the end of civilization or any dramatic problems — it would be at most a local emergency. Soviet experiences indicate that even when military high-level radioactive waste is dumped untreated directly into a river which 25,000 people use for drinking water and fishing, the result is a few tens of excess cancer deaths.[39] This is of course bad, but not that scary for an absolute worst-case scenario.
Early repositories for low and intermediate level waste constructed in Germany were badly designed and contained water-soluble waste. As a result, they have developed leaks, and expensive remediation efforts had to be undertaken.[40] So far there was no impact on the surrounding population. These repositories do not contain any spent fuel. Newer repositories are more carefully designed to prevent groundwater intrusion.
Note that nuclear waste disposal facilities are needed regardless of whether nuclear power is used. Many modern medical procedures and industrial processes require the use of radioactive substances, and as a consequence every industrialized country will generate some nuclear waste. Several countries with an energy policy that excludes nuclear power (notably Austria) operate research reactors and therefore generate a small quantity of high level waste. These countries will still eventually have to build high level waste repositories, but won't be able to tax their nuclear industry to fund them.
What are the environmental impacts of a nuclear power plant?
The primary impacts of a normally functioning nuclear power plant are associated with its cooling requirements.
- When cooling towers are used, substantial amounts of water are lost as steam.
- When open cycle cooling is used, the temperature of the nearby body of water can rise noticeably, which can be harmful to its ecosystem. If the regulations specify an upper limit for water temperature, the reactor may need to reduce power or cease operation in hot weather.
- Withdrawing water can kill fish and other aquatic organisms, unless water intakes are properly designed.
There are also some very low probability impacts:
- Release of a large quantity of radioactive substances into the environment during an accident. This happens extremely rarely (so far, it happened only 2 times: Chernobyl and Fukushima I) and is primarily a problem for human populations. Wildlife is affected only in the short term.[41] The physical health impact of any known radioactive release is limited by the fact that radiation is easy to detect and avoid.[42] However, psychological consequences and the resulting economic disruption are severe, as seen after the two aforementioned accidents. Addressing the widespread fear of radiation might reduce them to some extent. See the answer to this question.
- Leaks of nuclear waste from underground repositories. See the previous question for a discussion of this risk.
Do nuclear power plants emit radioactive substances into the environment?

Yes, a nuclear power plant emits a tiny amount of radioactive gases from the fission of fuel through a small smokestack-like structure near the reactor containment building. The additional dose from these releases to people living nearby is insignificant compared to variations in natural background radiation, which do not have any health impact. An equivalent coal power plant emits several times more radioactive material into the environment. The big hyperboloid towers that are stereotypically associated with nuclear power plants are cooling towers, which emit nothing but pure H2O (with very low and completely natural Deuterium admixtures).
Certain old reactors — for example, some of the British Magnox reactors — had limited shielding, causing direct gamma irradiation of their surroundings. In the case of the Dungeness reactor (now shut down), the extra dose could be as high as 550 µSv, though it was still below the legal limit of 1 mSv.[43]
Are nuclear power plants insured against accidents?
Yes. In many countries, a nuclear power plant is required to carry liability insurance up to a legally defined level, above which further damages are paid by the government. For example, in the U.S. there are three tiers of insurance. The first tier is $375 million of insurance coverage for each reactor. The second tier is a $12.6 billion insurance pool shared between all nuclear operators. The third tier is the government. If an accident happens, damages are first covered from the individual insurance. Damages above $375 million are satisfied from the shared pool. Finally, damages above ~$13 billion are covered by the government. In return, the insurance is no-fault, i.e. claims against the insurance are honoured even if the operator was not responsible for the accident.[44]
The above system does create a possibility that the costs of an extremely large nuclear accident might be shouldered by the taxpayers. This is the case for any very large industrial accident, such as a dam break, a refinery fire or a chemical spill. The insurance requirements are actually higher for nuclear power than for any other industry. For example, dams in the U.S. are not legally required to carry any insurance against catastrophic failure.[45][46] Fossil fuel power plants, oil rigs, fly ash ponds, etc. are rarely insured against the environmental pollution they can and do cause.
There is a common myth that no commercial insurance provider will cover nuclear power plants, which supposedly demonstrates that people "in the know" consider them very risky. This is obviously false.[47]
What are the potential consequences of a nuclear accident?
State-of-the-art analyses predict that an unmitigated accident in a light water reactor
This statement squares fairly well with the effects of some (in)famous accidents:
- Chernobyl killed 28 people through radiation poisoning, 2 directly by the explosion, and 15 through thyroid cancer. There were also 19 deaths among emergency response workers until 2004 from various causes, which gives a grand total of 64 deaths. The accident is expected to eventually cause 4000 cancer fatalities based on linear no-threshold modelling, which corresponds to an increase of a few percent in the cancer rate of the most exposed people.[49] This type of accident is not possible in reactors of different design, and the remaining Chernobyl-type reactors have been modified to prevent a similar scenario from reoccurring.
- Fukushima caused zero radiation-related deaths and the number of cancer deaths is expected to be statistically negligible.
- The Three Mile Island accident caused zero deaths and zero cancers.
However, this is only half of the story. While nuclear accidents kill hardly anybody, their most harmful effects are psychological in nature and caused by the fear of radiation rather than radiation itself.[50] They also have a substantial economic impact resulting from bad decisions (e.g. excessive mandatory evacuations) and the high cost of fossil fuels needed to replace lost generating capacity. Fortunately, radiophobia is not a physical law of nature, but a cultural contingency. Psychological effects of accidents can be significantly reduced by better education and rooting out these irrational fears.
Nuclear accidents have minimal long-term effects on wildlife. Their adverse impacts are far outweighed by reduced human activity in the affected area, as evidenced by the recovery of nature in the Chernobyl exclusion zone.[41][51] This prompted some people to semi-seriously explore the idea of deliberately spreading nuclear waste in valuable ecosystems to protect them from humans.[52]
Is there enough uranium?
- See also: Peak uranium
Known reserves of economically recoverable uranium amount to 7 million tons. This is sufficient for over 100 years of current consumption. Between 2009 and 2011, identified reserves increased by 12%, reflecting increased exploration.[53]
Because the price of natural uranium is only a small part of the cost of nuclear electricity (less than 5%), even large increases in uranium prices do not substantially impact the economics of nuclear power. If prices rise enough, it might become profitable to extract uranium from seawater, where it is present at a concentration of 3 parts per billion. Seawater extraction of uranium was demonstrated experimentally in Japan and the cost is expected to be in the $300-400/kg range, comparable to the highest historical price of $300/kg in 2009. However, no large scale facility was built to date.[54][55]
Breeder reactors are experimental reactors that can efficiently transform uranium-238 or thorium into plutonium or uranium-233, producing more fissile material than they consume. Widespread use of this technology would make nuclear power 100 times more fuel-efficient and effectively renewable: nuclear fuel would be constantly replenished from a pool of starting materials which is so large as to be practically inexhaustible.[56] However, Breeder reactors are not economical at current Uranium prices and have proven politically unfeasible in the past.
What are the impacts of uranium mining?
First, a little introduction. Uranium ore contains uranium as well as its decay products. Uranium itself is only weakly radioactive, but is a chemical hazard, with toxicity similar to lead. The decay products are significantly more radioactive and emit radon gas over time. Most of the uranium is contained in yellowcake, which leaves the mine as its primary output, while the decay products end up in the tailings. To minimize the emission of radon, these tailings are typically kept in large ponds covered with water. Once the mine is exhausted, the ponds are covered with a two meter layer of clay and topsoil.[57]
Now the impacts. Mismanagement of the tailings ponds may cause pollution of the groundwater with radioactive elements. If adequate ventilation is not provided in underground mines, workers are at an increased risk of lung cancer due to build-up of radon. This was the case in the '50s in the United States, where early miners from the Navajo tribe were the most affected.[58][59] In open cut mines, workers are at a risk from dust, which must be suppressed. These risks are similar to the risk from other mines, which can also contain significant quantities of radioactive elements, toxic metals, etc. Overall, the environmental impact of properly done uranium mining is similar to the impact of mining of other heavy metals. The footprint of an uranium mine is considerably lower than of a coal mine supplying a comparable amount of primary energy.[60]
Historically most uranium mines were open cut mines, which means a giant hole was dug in the ground to remove the ore. Recently there is an increase in the number of mines using in-situ leaching, which extracts uranium by pumping a solution of sodium bicarbonate or sulfuric acid into the ground and removing it after it reacts with the ore. This method is usually less expensive and avoids the disruption of the area, though it can result in groundwater pollution. This can be fixed after the mine is exhausted by flushing the deposit with neutralizing agents and clean water. (Note that the groundwater at such sites is typically not potable in the first place.) In 2011, 45% of uranium mined in the world came from ISL operations.[61] While the ISL method may sound similar to fracking used in shale gas extraction, the rocks are not fractured and the deposit must already be water-permeable to be compatible with this method.
The need for uranium mining may be reduced in the future through the use of breeder reactors, which can utilize the very large, already mined stocks of depleted uranium.
Can nuclear power be built fast enough to mitigate global warming?
Yes, at least on the technical level. To replace all fossil fuel electricity with nuclear power and not be late, the world would need to construct 3000 new reactors over 60 years, which is equivalent to 50 GW per year or one new 1 GW reactor per week. The highest historical rate of construction was 34 GW per year, in the mid-'80s.[62]
Can nuclear fusion be used to generate electric power?
Theoretically, yes. However, no fusion reactors have yet been built that generate more energy than they consume.
Nuclear fusion holds much promise as an energy source, but the practical problems involved in harnessing it are daunting. Unfortunately, this has resulted in a good deal of fusion woo, the most egregious example of which is cold fusion.
It is worth noting that the approaches to fusion power that have the highest chance of actually working would not completely prevent the creation of nuclear waste, since the nuclear reactions they are based on generate considerable amounts of high energy neutrons. These neutrons would make the components of the fusion reactor highly radioactive.
Nuclear weapons
How does a nuclear bomb work?
A nuclear bomb works by quickly rearranging a piece of highly concentrated fissile material into a configuration in which a nuclear chain reaction can quickly consume a substantial portion of it, then initiating the chain reaction with a burst of neutrons. The most common design is to surround the material with explosive lenses. When the lenses are detonated, they compress the core, increasing its density and allowing the chain reaction to achieve criticality.
The detailed schematics of nuclear weapons are highly classified in every country that produces them. As such, public knowledge about how nuclear weapons work is consists mainly of educated guesses of civilian nuclear physicists and engineers, declassified military information and leaks.
What is required to build a nuclear bomb?
On the technical level, there are four critical components required to build a working nuclear weapon:
- Weapons-grade fissile material.
- Know-how.
- Industrial infrastructure.
- Political support.
Some of the information required to construct a nuclear bomb is public knowledge, but obviously there are no detailed schematics available to download from BringYourOwnBomb.org, so any nuclear weapons program will by necessity include a research component. The industrial infrastructure required to manufacture electronics and conventional explosives for the bomb is available in most of the industrialized countries and also some developing ones. Obtaining the fissile material is the 'hard' technological part. Suitable materials include uranium-235 (at least 80% pure), plutonium-239 and uranium-233. Uranium-235 can be separated from natural uranium through uranium enrichment. Plutonium can be produced from uranium-238 in a purpose-built nuclear reactor. Uranium-233 can be produced from thorium in the same manner. The production of fissile material is hard to conduct in complete secrecy. Only Israel, with substantial help from France, was successful in keeping it a secret. North Korea's and Iran's nuclear weapons programs were public knowledge well before any bombs were made, and Iran was eventually persuaded to effectively abandon it.
The primary aspect that limits the spread of nuclear weapons is actually the lack of political support. Several countries have the technical and intellectual means to construct the bomb within a few years, but their populations and governments have no intention of doing so.
Can nuclear power infrastructure be used to create a nuclear weapon?
Some elements of nuclear power infrastructure can be used in a weapons program. However, it is difficult to do so clandestinely, in a way that does not alert the inspectors of the International Atomic Energy Agency (IAEA). The proliferating country could deny IAEA inspections, but even that would already send a message that something funky is going on. Most countries that have civilian nuclear facilities either already have nuclear weapons (e.g. France, China, India) or are uninterested in acquiring them (e.g. Spain, Mexico, Hungary).
Uranium enrichment plants can be used to produce high enriched (weapons-grade) uranium, suitable for use in bombs. This would be easy to detect, as production of weapons-grade uranium requires significant reconfiguration of the plant and causes a substantial decrease in the output of its normal product.[63]
Plutonium production is possible in reactors which allow the insertion and removal of objects into the core without turning off the reactor. These include reactors with online refueling capabilities, such as CANDU and RBMK, and research reactors. Production of weapons grade plutonium in light water reactors is impossible with anything resembling a normal fuel cycle due to inherent contamination with non-fissile plutonium-240.[64] Obtaining high-quality plutonium sufficient for one bomb requires processing 20 tons of irradiated uranium-238 rods in a dedicated chemical plant, which is somewhat hard to hide.
Given all of the above, creating a nuclear weapon clandestinely using existing civilian infrastructure has a dubious value proposition. It would be easier and less risky to build separate secret facilities, which was the route taken by Israel and North Korea. Most nuclear nonproliferation experts do not consider IAEA-inspected civilian nuclear facilities to pose any proliferation risk.[65]
How does a hydrogen bomb work?
A hydrogen bomb, also known as a thermonuclear weapon, uses the pressures and temperatures generated by a nuclear fission bomb to ignite the fusion of deuterium and tritium.[66]
The nuclear fusion reaction can:
- Provide back-pressure on the fission core, keeping it together longer (a simple design known as "boosted fission" or "boosted fusion" -- note the tritium gas in the diagram to the right);
- Provide the main explosive power of the weapon; and/or
- Compress a larger secondary fission warhead, triggering a much larger fission reaction than would have been possible with a regular explosive lens (known as the Teller-Ulam design).
Nuclear weapons that incorporate fusion tend to have much higher explosive yields than those that don't. Using fusion is the only way to get a yield in the megaton range, other than building a bomb so large that it can't be lifted by a bomber or an ICBM.
It is theoretically possible to build a thermonuclear weapon with no fission primary, known as a pure fusion weapon. However, due to current technological limitations, all designs proposed so far have been completely impractical.
What are the physical effects of a nuclear explosion?
There are four significant effects:[67]
- Fireball. Objects in the closest proximity to the explosion, including the bomb itself, are turned into plasma and completely disintegrated.
- Flash of electromagnetic radiation. An intense burst of infrared, visible and UV photons heats up objects and starts fires.
- Shock wave. An extremely powerful displacement of air destroys buildings.
- Radioactive fallout. Fission products and material irradiated by neutrons are dispersed into the air as radioactive dust, which settles near the explosion site.
What are the health effects of a nuclear explosion?
These effects are known from the studies of survivors of atomic bombings of Japan in World War II. The major short-term causes of death during the nuclear attack were:
- Flash burns (20-30% of total deaths)
- Radiation sickness (15-20%)
- Various injuries compounded by illness (50-60%)
In addition, the bombs have late effects. The risk associated with them is small compared to the risk of short-term death from the explosion.
- Survivors have an increased chance of cancer. Until 1998, there were around 1900 cases of cancer attributable to the bombings, not all of them fatal. This represents 11% of total cancers among exposed survivors.[68] Late deaths represent around 1% of the total deaths caused by the atomic bombings.
- There is no evidence of genetic damage, birth defects, or any other effects on the offspring of survivors.[5]
Since the Japanese bombings were both air burst explosions (the bomb exploded high above the ground), the effects of ground bursts in urban areas are unknown. A ground burst would cause more damage to buildings by creating seismic waves and generate more radioactive fallout, but the range of its shock wave would be shorter.
Is a nuclear bomb radioactive?
Yes, a nuclear bomb is weakly radioactive due to its plutonium or uranium content. The radiation is however too weak to cause symptoms of radiation poisoning. The movie gimmick where a stolen nuclear bomb is traced by finding terrorists who suffer from radiation sickness would not work in reality. Nuclear ballistic submarine crews spend months close to several nuclear bombs and a working reactor without any ill effects.
Would a nuclear bomb explode when disturbed?
No. Nuclear bombs are deliberately designed not to explode in fires, when shot at or when mechanically damaged. Even if these precautions fail, all current bombs are of the implosion type, where several explosive detonators and a neutron initiator must be fired at precise moments to cause a nuclear explosion. Any random disturbance to the weapon would be extremely unlikely to activate these components at the required times.
Early weapons used the gun method, where two subcritical masses of uranium-235 are brought together into a critical mass using explosives. Additionally, the Violet Club
Does a nuclear explosion make the area radioactive?
The strength of residual radiation highly depends on the manner in which the bomb is used. There is always a small amount of radioactive fallout from the fission products of the bomb itself. If the bomb explodes high in the air (air burst), as was done in the case of the atomic bombings of Japan, the amount of additional fallout is small and has no long-term impact. If the bomb explodes at surface level (ground burst), the displaced soil becomes radioactive from neutrons generated by the bomb, and the amount of fallout is far higher.[69]
Other nuclear technology
Is food irradiation safe?
- Main article: Food irradiation
Unequivocally yes. Food irradiation makes food safer and extends its shelf life. Irradiated food does not become radioactive. Fewer micronutrients are destroyed by food irradiation than are destroyed by the more conventional method of pasteurization, and pasteurization does little to reduce the nutritional value of foods very much in the first place. You can think of it as shining "light" upon your food to make last a while longer. After all, the radiation can be X-rays, and gamma-rays, which are electromagnetic radiation, just like light.
Are depleted uranium munitions dangerous?
- Main article: Depleted uranium
Depleted uranium (DU) is used in some armor-piercing tank shells and aircraft guns. Naturally they are dangerous as a weapon.[70] But you probably mean something different, something along the lines of "are they super poisonous and kill me on contact?" The answer is no. Dying from exposure to depleted uranium is highly improbable, unless someone shoots it at you.
DU is a chemical hazard to the kidneys (upon ingestion) and a minor radiological hazard to the lungs (upon inhalation as dust). DU-containing dust has very high density and quickly settles. DU has detectable impact on health only when you are hit with shrapnel or breathe dust near an attack site, and even then it is moderate.[71][72] Shrapnel from alternatives, such as tungsten-based alloys, is considerably more dangerous: 100% of rats implanted with pellets of such alloys develop extremely aggressive cancers within 4-5 months.[73]
Are whole-body scanners at airports a radiation risk?
No. Only one of the scanner technologies in use — backscatter X-ray — causes any radiation exposure. The dose received from this type of scanner is much lower than the extra dose received during the flight itself from increased cosmic radiation and is negligible even for frequent flyers.[74] The real problem with these scanners is that they are an invasion of privacy and part of the security theater.
See also
- Breeder reactor
- Depleted uranium
- Fear of radiation
- Food irradiation
External links
- NUKEMAP: An interactive web application that shows the range of effects of nuclear explosions on a Google map of your area.
References
- There are some rare exceptions, such as a very small variation in the rate of electron capture
File:Wikipedia's W.svg depending on pressure, but they are not important for practical purposes. - BBC: GCSE Bitesize - Background radiation
- Scientific American: The Workings of an Ancient Nuclear Reactor
- Jefferson Lab Q&A: Do radioactive things glow in the dark?
- Radiation Effects Research Fundation: Frequently asked question #7
- GreenFacts.org: Chernobyl: Have there been or will there be any effects on reproduction?
- U.S. NRC: Backgrounder on Dirty Bombs
- ARPANSA: Ionising Radiation and Health: Exposure Limits
- Health Physics Society: Emergency operations
- New York Times: Useful Mutants, Bred With Radiation
- Radiation Biology, section "In-utero Radiation Health Effects"
- Radiation Biology, section "Radiation Induced Genetic Damage"
- A reactor producing 1 GW of electricity has a thermal power of around 3 GW. An electric kettle is typically 2 kW.
- Clean Energy Insight: Comparing Industry Safety
- Next Big Future: Deaths per TWh by energy source
- David MacKay, Sustainable Energy – without hot air, p. 168
- N. Starfelt, C.-E. Wikdahl, [http://manhaz.cyf.gov.pl/manhaz/strona_konferencja_EAE-2001/15%20-%20Polenp~1.pdf Economic Analysis of Various Options of Electricity Generation - Taking into Account Health and Environmental Effects]
- A. Markandya, P. Wilkinson, Electricity generation and health Lancet 2007, 370 (9591), pp. 979-990
- European Commission, External Costs: Research results on socio-environmental damages due to electricity and transport, p. 15
- IAEA: Defence in depth in nuclear safety (INSAG-10)
- Video of an F-4 fighter plane crashing into a nuclear containment building wall
- ENEA - Preliminary analysis of an aircraft impact
- Note in any case that those two are among the largest planes in existence
- Congressional Research Service - Nuclear Power Plant Security and Vulnerabilities
- See: Chaïm Nissim
File:Wikipedia's W.svg - World Nuclear News: Security stops explosives at Ringhals
- Greenpeace activist paraglides into French nuclear plant
- Greenpeace activists break into French nuclear plant
- http://www.nytimes.com/2002/01/21/opinion/nuclear-reactors-as-terrorist-targets.html
- http://www.hiroshimasyndrome.com/the-uranium-explosive-myth.html
- World Nuclear Association: Radioactive Waste Management
- http://skeptics.stackexchange.com/a/5077/155
- The calculation was done based on the total electricity consumption, life expectancy and population of the UK, as well as the waste volume data given above. It is assumed that the given waste volumes refer to a reactor that operates at 90% capacity factor, and that waste production is proportional to electricity production. Milk carton = 1 l, soda bottle = 2 l, coffee cup = 200 ml, wine bottle = 750 ml
- David MacKay: Sustainable energy – without the hot air, p. 170
- UK Nuclear Decommissioning Authority: How is radioactive waste managed now - Intermediate Level Waste (ILW)
- Low Level Waste Repository Ltd: Repository Operations
- Bernard L. Cohen: The Nuclear Energy Option, chapter 11, see Fig. 1
- David J. Mossman et al., Carbonaceous substances in Oklo reactors—Analogue for permanent deep geologic disposal of anthropogenic nuclear waste. Reviews in Engineering Geology 2008, 19, p. 1-13
- Radioactive Contamination of the Techa River and its Effects
- See: Asse II mine,
File:Wikipedia's W.svg Morsleben radioactive waste repositoryFile:Wikipedia's W.svg - BBC News Europe: Wildlife defies Chernobyl radiation
- Radiation detectors capable of detecting single particles start at $200. Example Amazon listing
- British Nuclear Fuels Ltd. Discharges and Monitoring of the Environment in the UK Annual Report 2002, page 121, table 57
- U.S. NRC: Fact Sheet on Nuclear Insurance and Disaster Relief Funds
- Availability of Dam Insurance: A Report to Congress
- Nuclear Energy Institute - Price-Anderson Act fact sheet
- Brave New Climate: Nuclear risk insurance
- U.S. NRC: State-of-the-Art Reactor Consequence Analyses (SOARCA)
- GreenFacts: Health effects of the Chernobyl accident
- E. J. Bromet, J. M. Havenaar, Psychological and perceived health effects of the Chernobyl disaster: a 20-year review Health Physics 2007, 93(5), 516-521
- There is a Canadian scientist called Timothy Mousseau who apparently dedicated his career to contesting this idea. He investigates environmental indicators which are irrelevant or which tend to be "better" in areas with significant human activity and claims that this proves an ecological breakdown. Anti-nuclear sites and "balanced" news articles almost always refer to his work when discussing this subject. His conclusions are unsupported, since more relevant indicators — such as the population of apex predators — indicate that the Chernobyl exclusion zone is a thriving ecosystem.
- Treehugger: Nuclear Waste Can Protect Tropical Forests
- World Nuclear News: Uranium supplies good for the long haul
- David MacKay, Sustainable Energy - without the hot air, p. 163
- Masao Tamada, Current status of technology for collection of uranium from seawater
- Bernard L. Cohen, Breeder reactors: A renewable energy source. American Journal of Physics January 1983, 51(1), p. 75–76. doi:10.1119/1.13440.
- World Nuclear Association: Environmental Aspects of Uranium Mining
- L.S. Gottlieb, L.A. Husen, Lung cancer among Navajo uranium miners. CHEST Journal, 1982, 81(4), p. 449-452
- R.J. Roscoe et al., Mortality among Navajo uranium miners. American Journal of Public Health, 1995, 85(4), p. 535-540
- The environmental footprints of coal and uranium mining
- World Nuclear Association: In Situ Leach (ISL) Mining of Uranium
- David MacKay, Sustainable energy - without the hot air, p. 171
- Safeguards Techniques and Equipment: 2011 Edition, International Atomic Energy Agency - contains detailed descriptions of the equipment used to ensure the absence of highly enriched uranium in inspected enrichment facilities.
- http://depletedcranium.com/why-you-cant-build-a-bomb-from-spent-fuel/
- R. Scott Kemp, Environmental Detection of Clandestine Nuclear Weapon Programs,
- Often lithium-6, which generates tritium when irradiated with high energy neutrons, is used instead of tritium because it's easier to handle and doesn't need to be replaced every few years.
- Atomic Archive: The Effects of Nuclear Weapons
- Radiation Effects Research Fundation: Frequently asked question #2
- Atomic Archive: Effects of Nuclear Weapons: Radioactive Fallout
- Illustrative example
- World Health Organization: Depleted uranium: sources, exposure and health effects
- Health Physics Society fact sheet on DU
- Embedded weapons-grade tungsten alloy shrapnel rapidly induces metastatic high-grade rhabdomyosarcomas in F344 rats
- P. Mehta, R. Smith-Bindman, Airport full-body screening: what is the risk? Archives of Internal Medicine 2011, 171(12), 1112-1115