Background and Identification
The Respironics BiPaP Vision (Model Number: 582059) was reviewed by the United States FDA in 1998, and available for purchase soon after. Though available for purchase from online retailers, the clinical manual recommends that a certified and trained physician first conducts tests on the patient before the machine’s settings are to be set, implying that the BiPaP is to be used by medical professionals.
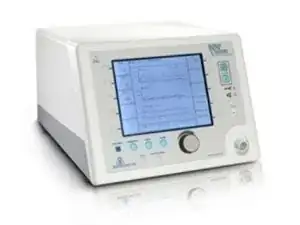
The main part of the Respironics BiPaP Vision is the main console – a beige-colored box with a blue LED screen and buttons on the front. Besides the console, the Respironics BiPaP Vision includes a stand that provides the machine with mobility, a lightweight alarm control, and various pumps/inserts for the use of the machine’s main ventilator functions.
This device is no longer manufactured or supported by Philips/Respironics, as indicated by an “End of Life Letter” released for the product in the past; that document is not accessible for online viewing. The Respironics BiPaP Vision was likely phased out because of the popularization of modern lightweight/more technologically advanced ventilators, as may be seen on the Philips website.
Nowadays, depending on the vendor, the device may sell anywhere from a few hundred dollars to more than one thousand.
The model number and serial number (s/n) for this device may be found on the backside of this device, in the bottom left-hand corner.
Technical Specifications
Physical
- Dimensions: 40.6 x 36.5 x 27cm
- Weight: 15.4kg
- Display: LCD video screen
Electrical
- AC Voltage: 100/120/230/240 V~
- AC Frequency: 50/60 Hz
- AC Current: 3.0 A Maximum
Nurse Call/Remote Alarm Connecter
- Voltage: 50 VDC or 25 VAC maximum
- Current: 1 Amp maximum